A heterogeneous population of nuclear-encoded mitochondrial mRNAs is present in the axons of primary sympathetic neurons
- PMID: 27318271
- PMCID: PMC5023477
- DOI: 10.1016/j.mito.2016.06.002
A heterogeneous population of nuclear-encoded mitochondrial mRNAs is present in the axons of primary sympathetic neurons
Abstract
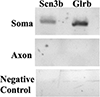
Mitochondria are enriched in subcellular regions of high energy consumption, such as axons and pre-synaptic nerve endings. Accumulating evidence suggests that mitochondrial maintenance in these distal structural/functional domains of the neuron depends on the "in-situ" translation of nuclear-encoded mitochondrial mRNAs. In support of this notion, we recently provided evidence for the axonal targeting of several nuclear-encoded mRNAs, such as cytochrome c oxidase, subunit 4 (COXIV) and ATP synthase, H+ transporting and mitochondrial Fo complex, subunit C1 (ATP5G1). Furthermore, we showed that axonal trafficking and local translation of these mRNAs plays a critical role in the generation of axonal ATP. Using a global gene expression analysis, this study identified a highly diverse population of nuclear-encoded mRNAs that were enriched in the axon and presynaptic nerve terminals. Among this population of mRNAs, fifty seven were found to be at least two-fold more abundant in distal axons, as compared with the parental cell bodies. Gene ontology analysis of the nuclear-encoded mitochondrial mRNAs suggested functions for these gene products in molecular and biological processes, including but not limited to oxidoreductase and electron carrier activity and proton transport. Based on these results, we postulate that local translation of nuclear-encoded mitochondrial mRNAs present in the axons may play an essential role in local energy production and maintenance of mitochondrial function.
Keywords: Campenot cell culture chambers; Microarray analyses; Subcellular localization; Superior cervical ganglion; mRNAs.
Published by Elsevier B.V.
Figures



References
-
- Aschrafi A, Schwechter AD, Mameza MG, Natera-Naranjo O, Gioio AE, Kaplan BB. MicroRNA-338 regulates local cytochrome c oxidase IV mRNA levels and oxidative phosphorylation in the axons of sympathetic neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:12581–12590. - PMC - PubMed
-
- Gioio AE, Eyman M, Zhang H, Lavina ZS, Giuditta A, Kaplan BB. Local synthesis of nuclear-encoded mitochondrial proteins in the presynaptic nerve terminal. J. Neurosci. Res. 2001;64:447–453. - PubMed
-
- Goes FS, Hamshere ML, Seifuddin F, Pirooznia M, Belmonte-Mahon P, Breuer R, Schulze T, Nothen M, Cichon S, Rietschel M, Holmans P, Zandi PP, Bipolar Genome S, Craddock N, Potash JB. Genome-wide association of mood-incongruent psychotic bipolar disorder. Transl Psychiatry. 2012;2:e180. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

