Dendritic cell maturation and cross-presentation: timing matters!
- PMID: 27319345
- PMCID: PMC6680313
- DOI: 10.1111/imr.12432
Dendritic cell maturation and cross-presentation: timing matters!
Abstract
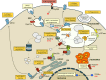
As a population, dendritic cells (DCs) appear to be the best cross-presenters of internalized antigens on major histocompatibility complex class I molecules in the mouse. To do this, DCs have developed a number of unique and dedicated means to control their endocytic and phagocytic pathways: among them, the capacity to limit acidification of their phagosomes, to prevent proteolytic degradation, to delay fusion of phagosomes to lysosomes, to recruit ER proteins to phagosomes, and to export phagocytosed antigens to the cytosol. The regulation of phagocytic functions, and thereby of antigen processing and presentation by innate signaling, represents a critical level of integration of adaptive and innate immune responses. Understanding how innate signals control antigen cross-presentation is critical to define effective vaccination strategies for CD8(+) T-cell responses.
Keywords: cross-presentation; dendritic cell; dendritic cells maturation; phagocytosis; toll-like receptor.
© 2016 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures
References
-
- Savina A, Amigorena S. Phagocytosis and antigen presentation in dendritic cells. Immunol Rev 2007;219:143–156. - PubMed
-
- Joffre OP, Segura E, Savina A, Amigorena S. Cross‐presentation by dendritic cells. Nat Rev Immunol 2012;12:557–569. - PubMed
-
- Shen L, Sigal LJ, Boes M, Rock KL. Important role of cathepsin S in generating peptides for TAP‐independent MHC class I crosspresentation in vivo. Immunity 2004;21:155–165. - PubMed
-
- Bertholet S, et al. Leishmania antigens are presented to CD8+ T cells by a transporter associated with antigen processing‐independent pathway in vitro and in vivo. J Immunol 2006;177:3525–3533. - PubMed
-
- Kovacsovics‐Bankowski M, Rock KL. A phagosome‐to‐cytosol pathway for exogenous antigens presented on MHC class I molecules. Science 1995;267:243–246. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

