Androgen receptor transcriptionally regulates μ-opioid receptor expression in rat trigeminal ganglia
- PMID: 27320211
- PMCID: PMC5498154
- DOI: 10.1016/j.neuroscience.2016.06.023
Androgen receptor transcriptionally regulates μ-opioid receptor expression in rat trigeminal ganglia
Abstract
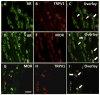
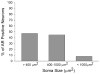
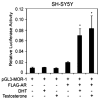
The involvement of testosterone in pain, inflammation, and analgesia has been reported, but the role of androgen receptor (AR), a steroid receptor for testosterone, is not well understood. We have previously shown that peripheral inflammation upregulates μ-opioid receptor (MOR) in rat trigeminal ganglia (TG) in a testosterone-dependent manner. In this study, we hypothesized that testosterone regulates MOR expression via transcriptional activities of AR in TG. We first examined whether AR is co-expressed with MOR in TG neurons. Our immunohistochemical experiment revealed that AR staining is detected in neurons of all sizes in TG and that a subset of AR is expressed in MOR as well as in TRPV1-positive neurons. We identified the promoter region of the rat MOR gene contains putative AR binding sites. Using chromatin immunoprecipitation assay, we demonstrated that AR directly binds to these sites in TG extracts. We confirmed with luciferase reporter assay that AR activated the MOR promoter in response to androgens in a human neuroblastoma cell line (5H-5YSY). These data demonstrated that AR functions as a transcriptional regulator of the MOR gene activity. Finally, we showed that flutamide, a specific AR antagonist, prevents complete Freund's adjuvant (CFA)-induced upregulation of MOR mRNA in TG, and that flutamide dose-dependently blocks the efficacy of DAMGO, a specific MOR agonist, on CFA-induced mechanical hypersensitivity. Our results expand the knowledge regarding the role of androgens and their receptor in pain and analgesia and have important clinical implications, particularly for inflammatory pain patients with low or compromised plasma testosterone levels.
Keywords: flutamide; inflammation; peripheral; sensory neurons; testosterone.
Copyright © 2016 IBRO. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
There are no conflicts of interest associated with the present study
Figures


Similar articles
-
Sex differences in peripheral mu-opioid receptor mediated analgesia in rat orofacial persistent pain model.PLoS One. 2015 Mar 25;10(3):e0122924. doi: 10.1371/journal.pone.0122924. eCollection 2015. PLoS One. 2015. PMID: 25807259 Free PMC article.
-
Role of peripheral mu-opioid receptors in inflammatory orofacial muscle pain.Neuroscience. 2007 May 25;146(3):1346-54. doi: 10.1016/j.neuroscience.2007.02.024. Epub 2007 Mar 26. Neuroscience. 2007. PMID: 17379421
-
Sex differences in μ-opioid receptor expression in trigeminal ganglia under a myositis condition in rats.Eur J Pain. 2014 Feb;18(2):151-61. doi: 10.1002/j.1532-2149.2013.00352.x. Epub 2013 Jun 25. Eur J Pain. 2014. PMID: 23801566 Free PMC article.
-
The mechanism of μ-opioid receptor (MOR)-TRPV1 crosstalk in TRPV1 activation involves morphine anti-nociception, tolerance and dependence.Channels (Austin). 2015;9(5):235-43. doi: 10.1080/19336950.2015.1069450. Epub 2015 Jul 15. Channels (Austin). 2015. PMID: 26176938 Free PMC article. Review.
-
Opioid-induced redistribution of 6TM and 7TM μ opioid receptors: A hypothesized mechanistic facilitator model of opioid-induced hyperalgesia.Pharmacol Rep. 2016 Aug;68(4):686-91. doi: 10.1016/j.pharep.2016.03.003. Epub 2016 Mar 19. Pharmacol Rep. 2016. PMID: 27116700 Review.
Cited by
-
Modulatory Processes in Craniofacial Pain States.Adv Neurobiol. 2024;35:107-124. doi: 10.1007/978-3-031-45493-6_6. Adv Neurobiol. 2024. PMID: 38874720
-
HPA axis dysfunction during morphine withdrawal in offspring of female rats exposed to opioids preconception.Neurosci Lett. 2022 Mar 16;773:136479. doi: 10.1016/j.neulet.2022.136479. Epub 2022 Jan 24. Neurosci Lett. 2022. PMID: 35085692 Free PMC article.
-
Effects of intramuscular morphine in men and women with temporomandibular disorder with myofascial pain.Oral Dis. 2018 Nov;24(8):1591-1598. doi: 10.1111/odi.12919. Epub 2018 Jul 6. Oral Dis. 2018. PMID: 29920852 Free PMC article. Clinical Trial.
-
The influence of sex on neuroimmune communication, pain, and physiology.Biol Sex Differ. 2024 Oct 22;15(1):82. doi: 10.1186/s13293-024-00660-w. Biol Sex Differ. 2024. PMID: 39439003 Free PMC article. Review.
-
Peripherally Acting Opioids in Orofacial Pain.Front Neurosci. 2021 May 4;15:665445. doi: 10.3389/fnins.2021.665445. eCollection 2021. Front Neurosci. 2021. PMID: 34017236 Free PMC article. Review.
References
-
- Ambalavanar R, Moutanni A, Dessem D. Inflammation of craniofacial muscle induces widespread mechanical allodynia. Neurosci Lett. 2006;399:249–254. - PubMed
-
- Araneo BA, Dowell T, Diegel M, Daynes RA. Dihydrotestosterone exerts a depressive influence on the production of interleukin-4 (IL-4), IL-5, and gamma-interferon, but not IL-2 by activated murine T cells. Blood. 1991;78:688–699. - PubMed
-
- Atanassova N, Koeva Y, Bakalska M, Pavlova E, Nikolov B, Davidoff M. Loss and recovery of androgen receptor protein expression in the adult rat testis following androgen withdrawal by ethane dimethanesulfonate. Folia Histochem Cytobiol. 2006;44:81–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

