Interplay between 15-lipoxygenase-1 and metastasis-associated antigen 1 in the metastatic potential of colorectal cancer
- PMID: 27320813
- PMCID: PMC6495825
- DOI: 10.1111/cpr.12267
Interplay between 15-lipoxygenase-1 and metastasis-associated antigen 1 in the metastatic potential of colorectal cancer
Abstract
Objectives: Metastasis-associated antigen 1 (MTA1) is implicated in metastasis while 15-lipoxygenase-1 (15-LOX-1) reduces cell motility, when re-expressed in colorectal cancer (CRC). We aimed to understand any potential interplay between MTA1 and 15-LOX-1 in CRC metastasis.
Materials and methods: ALOX15 and MTA1 expression in tumour and normal samples were analysed from TCGA RNA-seq data, microarray data sets and a human CRC cDNA array. Western blots, chromatin immunoprecipitation (ChIP), luciferase assays and electrophoretic mobility shift assays (EMSA) were carried out in HT-29 and LoVo cells re-expressing 15-LOX-1 to determine NF- κB activity at the MTA1 promoter. Functional assays in cells ectopically expressing either 15-LOX-1, MTA-1 or both, were carried out to determine adhesion and cell motility.
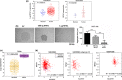
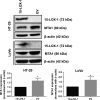
Results: Significantly higher expression of MTA1 was observed in tumours compared to normal tissues; MTA1 overexpression resulted in reduced adhesion in CRC cell lines. Re-expression of 15-LOX-1 in the CRC cell lines reduced expression of endogenous MTA1, corroborated by negative correlation between the two genes in two independent human CRC microarray data sets, with greater significance in specific subsets of patients. DNA binding and transcriptional activity of NF-κB at the MTA1 promoter was significantly lower in cells re-expressing 15-LOX-1. Functionally, the same cells had reduced motility, which was rescued when they overexpressed MTA1, and further corroborated by expressions of E-cadherin and vimentin.
Conclusions: Expression of MTA1 and 15-LOX-1 negatively correlated in specific subsets of CRC. Mechanistically, this is at least in part through reduced recruitment of NF-κB to the MTA1 promoter.
© 2016 John Wiley & Sons Ltd.
Figures



Similar articles
-
MTA-1 expression is associated with metastasis and epithelial to mesenchymal transition in colorectal cancer cells.Tumour Biol. 2013 Apr;34(2):1189-204. doi: 10.1007/s13277-013-0662-x. Epub 2013 Jan 31. Tumour Biol. 2013. PMID: 23371285
-
15-LOX-1 transcription suppression through the NuRD complex in colon cancer cells.Oncogene. 2009 Mar 26;28(12):1496-505. doi: 10.1038/onc.2008.494. Epub 2009 Feb 9. Oncogene. 2009. PMID: 19198625 Free PMC article.
-
MicroRNA-543 suppresses colorectal cancer growth and metastasis by targeting KRAS, MTA1 and HMGA2.Oncotarget. 2016 Apr 19;7(16):21825-39. doi: 10.18632/oncotarget.7989. Oncotarget. 2016. PMID: 26968810 Free PMC article.
-
Properties and clinical relevance of MTA1 protein in human cancer.Cancer Metastasis Rev. 2014 Dec;33(4):891-900. doi: 10.1007/s10555-014-9516-2. Cancer Metastasis Rev. 2014. PMID: 25359582 Review.
-
Identification and characterization of metastasis-associated gene/protein 1 (MTA1).Cancer Metastasis Rev. 2014 Dec;33(4):837-42. doi: 10.1007/s10555-014-9510-8. Cancer Metastasis Rev. 2014. PMID: 25315816 Review.
Cited by
-
Molecular Targets in Precision Chemoprevention of Colorectal Cancer: An Update from Pre-Clinical to Clinical Trials.Int J Mol Sci. 2020 Dec 17;21(24):9609. doi: 10.3390/ijms21249609. Int J Mol Sci. 2020. PMID: 33348563 Free PMC article. Review.
-
Opposing roles of the aldo-keto reductases AKR1B1 and AKR1B10 in colorectal cancer.Cell Oncol (Dordr). 2017 Dec;40(6):563-578. doi: 10.1007/s13402-017-0351-7. Epub 2017 Sep 19. Cell Oncol (Dordr). 2017. PMID: 28929377
-
Emerging cellular functions of the lipid metabolizing enzyme 15-Lipoxygenase-1.Cell Prolif. 2018 Oct;51(5):e12472. doi: 10.1111/cpr.12472. Epub 2018 Jul 30. Cell Prolif. 2018. PMID: 30062726 Free PMC article. Review.
-
ALOXE3 expression predicts poor prognosis and modulates immune infiltration in colon adenocarcinoma.World J Surg Oncol. 2025 Jul 23;23(1):296. doi: 10.1186/s12957-025-03939-3. World J Surg Oncol. 2025. PMID: 40696372 Free PMC article.
-
Exploiting the Acidic Extracellular pH: Evaluation of Streptococcus salivarius M18 Postbiotics to Target Cancer Cells.Probiotics Antimicrob Proteins. 2022 Dec;14(6):995-1011. doi: 10.1007/s12602-021-09806-3. Epub 2021 Jun 2. Probiotics Antimicrob Proteins. 2022. PMID: 34080175
References
-
- Toh Y, Nicolson GL. Properties and clinical relevance of MTA1 protein in human cancer. Cancer Metastasis Rev. 2014;33:891–900. - PubMed
-
- Mazumdar A, Wang RA, Mishra SK, et al. Transcriptional repression of oestrogen receptor by metastasis‐associated protein 1 corepressor. Nat Cell Biol. 2001;3:30–37. - PubMed
-
- Li DQ, Kumar R. Unravelling the complexity and functions of MTA Coregulators in Human Cancer. Adv Cancer Res. 2015;127:1–47. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

