Defects in the CAPN1 Gene Result in Alterations in Cerebellar Development and Cerebellar Ataxia in Mice and Humans
- PMID: 27320912
- PMCID: PMC4927383
- DOI: 10.1016/j.celrep.2016.05.044
Defects in the CAPN1 Gene Result in Alterations in Cerebellar Development and Cerebellar Ataxia in Mice and Humans
Abstract
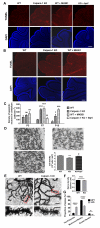
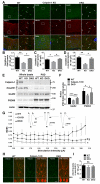
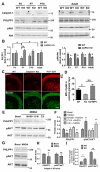
A CAPN1 missense mutation in Parson Russell Terrier dogs is associated with spinocerebellar ataxia. We now report that homozygous or heterozygous CAPN1-null mutations in humans result in cerebellar ataxia and limb spasticity in four independent pedigrees. Calpain-1 knockout (KO) mice also exhibit a mild form of ataxia due to abnormal cerebellar development, including enhanced neuronal apoptosis, decreased number of cerebellar granule cells, and altered synaptic transmission. Enhanced apoptosis is due to absence of calpain-1-mediated cleavage of PH domain and leucine-rich repeat protein phosphatase 1 (PHLPP1), which results in inhibition of the Akt pro-survival pathway in developing granule cells. Injection of neonatal mice with the indirect Akt activator, bisperoxovanadium, or crossing calpain-1 KO mice with PHLPP1 KO mice prevented increased postnatal cerebellar granule cell apoptosis and restored granule cell density and motor coordination in adult mice. Thus, mutations in CAPN1 are an additional cause of ataxia in mammals, including humans.
Keywords: apoptosis; ataxia; calpain-1; cerebellum; development.
Copyright © 2016 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Altman J. Postnatal development of the cerebellar cortex in the rat. 3. Maturation of the components of the granular layer. J Comp Neurol. 1972;145:465–513. - PubMed
-
- Amadoro G, Pieri M, Ciotti MT, Carunchio I, Canu N, Calissano P, Zona C, Severini C. Substance P provides neuroprotection in cerebellar granule cells through Akt and MAPK/Erk activation: evidence for the involvement of the delayed rectifier potassium current. Neuropharmacology. 2007;52:1366–1377. - PubMed
-
- Balazs R, Jorgensen OS, Hack N. N-methyl-D-aspartate promotes the survival of cerebellar granule cells in culture. Neuroscience. 1988;27:437–451. - PubMed
-
- Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, Shiloh Y, Crawley JN, Ried T, Tagle D, Wynshaw-Boris A. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell. 1996;86:159–171. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

