CRF-like receptor SEB-3 in sex-common interneurons potentiates stress handling and reproductive drive in C. elegans
- PMID: 27321013
- PMCID: PMC4915151
- DOI: 10.1038/ncomms11957
CRF-like receptor SEB-3 in sex-common interneurons potentiates stress handling and reproductive drive in C. elegans
Abstract
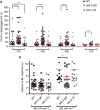
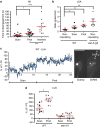
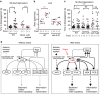
Environmental conditions can modulate innate behaviours. Although male Caenorhabditis elegans copulation can be perturbed in the presence of stress, the mechanisms underlying its decision to sustain copulation are unclear. Here we describe a mating interference assay, which quantifies the persistence of male C. elegans copulation in noxious blue light. We show that between copulations, the male escapes from blue light illumination at intensities over 370 μW mm(-2). This response is attenuated in mutants with constitutive activation of the corticotropin-releasing factor receptor family homologue SEB-3. We show that activation of this receptor causes sex-common glutamatergic lumbar ganglion interneurons (LUA) to potentiate downstream male-specific reproduction circuits, allowing copulatory behaviours to partially override the light-induced escape responses in the male. SEB-3 activation in LUA also potentiates copulation during mild starvation. We suggest that SEB-3 activation allows C. elegans to acclimate to the environment and thus continue to execute innate behaviours even under non-optimal conditions.
Figures






References
-
- de Wied D. & Gispen W. H. in Peptides in Neurobiology ed. Gainer H. 397–448Springer US (1977).
-
- Schally A. V. Aspects of hypothalamic regulation of the pituitary gland. Science 202, 18–28 (1978). - PubMed
-
- Hetherington A. W. & Ranson S. W. The spontaneous activity and food intake of rats with hypothalamic lesions. Am J Physiol. Legacy Content 136, 609–617 (1942).
-
- Britton K. T., Lee G., Dana R., Risch S. C. & Koob G. F. Activating and ‘anxiogenic' effects of corticotropin releasing factor are not inhibited by blockade of the pituitary-adrenal system with dexamethasone. Life Sci. 39, 1281–1286 (1986). - PubMed
-
- Müller M. B. et al. Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat. Neurosci. 6, 1100–1107 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

