Emerging Roles of Toxin-Antitoxin Modules in Bacterial Pathogenesis
- PMID: 27322231
- PMCID: PMC6273597
- DOI: 10.3390/molecules21060790
Emerging Roles of Toxin-Antitoxin Modules in Bacterial Pathogenesis
Abstract
Toxin-antitoxin (TA) cassettes are encoded widely by bacteria. The modules typically comprise a protein toxin and protein or RNA antitoxin that sequesters the toxin factor. Toxin activation in response to environmental cues or other stresses promotes a dampening of metabolism, most notably protein translation, which permits survival until conditions improve. Emerging evidence also implicates TAs in bacterial pathogenicity. Bacterial persistence involves entry into a transient semi-dormant state in which cells survive unfavorable conditions including killing by antibiotics, which is a significant clinical problem. TA complexes play a fundamental role in inducing persistence by downregulating cellular metabolism. Bacterial biofilms are important in numerous chronic inflammatory and infectious diseases and cause serious therapeutic problems due to their multidrug tolerance and resistance to host immune system actions. Multiple TAs influence biofilm formation through a network of interactions with other factors that mediate biofilm production and maintenance. Moreover, in view of their emerging contributions to bacterial virulence, TAs are potential targets for novel prophylactic and therapeutic approaches that are required urgently in an era of expanding antibiotic resistance. This review summarizes the emerging evidence that implicates TAs in the virulence profiles of a diverse range of key bacterial pathogens that trigger serious human disease.
Keywords: antibiotic resistance; biofilm formation; pathogenesis; persistence; toxin-antitoxin complexes; virulence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



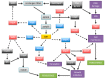
References
-
- Wang X., Quinn P.J. Endotoxins: Lipopolysaccharides of gram-negative bacteria. Subcell. Biochem. 2010;53:3–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

