Capsaicin, Nociception and Pain
- PMID: 27322240
- PMCID: PMC6273518
- DOI: 10.3390/molecules21060797
Capsaicin, Nociception and Pain
Abstract
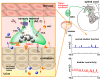
Capsaicin, the pungent ingredient of the hot chili pepper, is known to act on the transient receptor potential cation channel vanilloid subfamily member 1 (TRPV1). TRPV1 is involved in somatic and visceral peripheral inflammation, in the modulation of nociceptive inputs to spinal cord and brain stem centers, as well as the integration of diverse painful stimuli. In this review, we first describe the chemical and pharmacological properties of capsaicin and its derivatives in relation to their analgesic properties. We then consider the biochemical and functional characteristics of TRPV1, focusing on its distribution and biological effects within the somatosensory and viscerosensory nociceptive systems. Finally, we discuss the use of capsaicin as an agonist of TRPV1 to model acute inflammation in slices and other ex vivo preparations.
Keywords: TRPV1 receptor; analgesia; capsaicin; nociception; resinferatoxin; sensitization; somatic pain; vanilloids; visceral pain.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Thresh J.C. Isolation of capsacin. Pharm. J. Tr. 1876;6:941–947.
-
- Nelson E.K. The constitution of capsaicin, the pungent principle of capsicum. II. J. Am. Chem. Soc. 1920;42:597–599.
-
- De A.K., editor. Capsicum: The Genus Capsicum. CRC Press; London, UK: 2003.
-
- Musfiroh I., Mutakin M., Angelina T., Muchtaridi M. Capsaicin level of various capsicum fruits. Int. J. Pharm. Pharm. Sci. 2013;5:248–251.
-
- Caterina M.J., Schumacher M.A., Tominaga M., Rosen T.A., Levine J.D., Julius D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

