The mouse cortico-striatal projectome
- PMID: 27322419
- PMCID: PMC5564682
- DOI: 10.1038/nn.4332
The mouse cortico-striatal projectome
Abstract
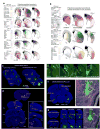
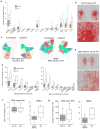
Different cortical areas are organized into distinct intracortical subnetworks. The manner in which descending pathways from the entire cortex interact subcortically as a network remains unclear. We developed an open-access comprehensive mesoscale mouse cortico-striatal projectome: a detailed connectivity projection map from the entire cerebral cortex to the dorsal striatum or caudoputamen (CP) in rodents. On the basis of these projections, we used new computational neuroanatomical tools to identify 29 distinct functional striatal domains. Furthermore, we characterized different cortico-striatal networks and how they reconfigure across the rostral-caudal extent of the CP. The workflow was also applied to select cortico-striatal connections in two different mouse models of disconnection syndromes to demonstrate its utility for characterizing circuitry-specific connectopathies. Together, our results provide the structural basis for studying the functional diversity of the dorsal striatum and disruptions of cortico-basal ganglia networks across a broad range of disorders.
Conflict of interest statement
The authors declare no competing financial interests.
Figures






References
-
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

