Activation of volume-sensitive Cl- channel mediates autophagy-related cell death in myocardial ischaemia/reperfusion injury
- PMID: 27322431
- PMCID: PMC5129937
- DOI: 10.18632/oncotarget.10050
Activation of volume-sensitive Cl- channel mediates autophagy-related cell death in myocardial ischaemia/reperfusion injury
Abstract
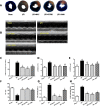
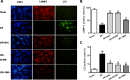
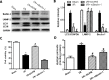
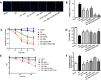
Excessive reactive oxygen species (ROS) plays an important role in myocardial ischemia/reperfusion (I/R) injury, which triggers not only myocardial cellular apoptosis but also autophagy-related cell death, in which volume-sensitive outwardly rectifying (VSOR) Cl- channel-activated by ROS contributes to cell apoptotic volume decrease, playing an incipient incident of cellular apoptosis. However, whether VSOR Cl- channel concurrently participates in autophagy-related cell death regulation remains unclear. To illuminate the issue, studies underwent in myocardial vitro and vivo I/R model. Rats were performed to ischemia 30 minutes and subsequent reperfusion 24-96 hours, ROS scavenger (NAC), VSOR Cl- channel blocker (DCPIB) and autophagy inhibitor (3MA) were administered respectively. Results showed that oxidative stress, LC3-II stain and inflammation in myocardial tissue were markedly increased, lysosome associated membrane protein-2 (LAMP2) were significantly reduced with I/R group as compared with sham group, reperfusion significantly led to damage in myocardial tissue and heart function, whereas the disorder could be rescued through these agents. Moreover, primary neonatal rat cardiomyocytes hypoxia/reoxygenation model were administered, results showed that VSOR Cl- channel-activated by reoxygenation could cause both cell volume decrease and intracellular acidification, which further increased LC3 and depleted of LAMP2, resulting in autophagy-related cell death. Interestingly, VSOR Cl- channel-blocked by DCPIB could stably maintain the cell volume, intracellular pH, abundant LAMP2 and autophagic intensity regardless of ROS intension derived from reoxygenation injury or adding H2O2. These results first demonstrate that VSOR Cl- channel-activated is a pivotal event to trigger autophagy-related death, which reveals a novel therapeutic target to decrease myocardial I/R injury.
Keywords: Pathology Section; VSOR Cl- channel; autophagy; cell death; ischemia/reperfusion injury; reactive oxygen species.
Conflict of interest statement
There is no conflict of interest.
Figures







Similar articles
-
Volume-sensitive outwardly rectifying chloride channel blockers protect against high glucose-induced apoptosis of cardiomyocytes via autophagy activation.Sci Rep. 2017 Mar 16;7:44265. doi: 10.1038/srep44265. Sci Rep. 2017. PMID: 28300155 Free PMC article.
-
Activation of volume-sensitive outwardly rectifying chloride channel by ROS contributes to ER stress and cardiac contractile dysfunction: involvement of CHOP through Wnt.Cell Death Dis. 2014 Nov 20;5(11):e1528. doi: 10.1038/cddis.2014.479. Cell Death Dis. 2014. PMID: 25412307 Free PMC article.
-
Chloride channel inhibition prevents ROS-dependent apoptosis induced by ischemia-reperfusion in mouse cardiomyocytes.Cell Physiol Biochem. 2005;16(4-6):147-54. doi: 10.1159/000089840. Cell Physiol Biochem. 2005. PMID: 16301815
-
Roles of volume-regulatory anion channels, VSOR and Maxi-Cl, in apoptosis, cisplatin resistance, necrosis, ischemic cell death, stroke and myocardial infarction.Curr Top Membr. 2019;83:205-283. doi: 10.1016/bs.ctm.2019.03.001. Epub 2019 Apr 19. Curr Top Membr. 2019. PMID: 31196606 Review.
-
Volume-sensitive chloride channels involved in apoptotic volume decrease and cell death.J Membr Biol. 2006 Jan;209(1):21-9. doi: 10.1007/s00232-005-0836-6. Epub 2006 Apr 17. J Membr Biol. 2006. PMID: 16685598 Review.
Cited by
-
Properties, Structures, and Physiological Roles of Three Types of Anion Channels Molecularly Identified in the 2010's.Front Physiol. 2021 Dec 23;12:805148. doi: 10.3389/fphys.2021.805148. eCollection 2021. Front Physiol. 2021. PMID: 35002778 Free PMC article. Review.
-
The Impact of Moderate Chronic Hypoxia and Hyperoxia on the Level of Apoptotic and Autophagic Proteins in Myocardial Tissue.Oxid Med Cell Longev. 2018 Aug 16;2018:5786742. doi: 10.1155/2018/5786742. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 30186545 Free PMC article.
-
LRRC8/VRAC channels exhibit a noncanonical permeability to glutathione, which modulates epithelial-to-mesenchymal transition (EMT).Cell Death Dis. 2019 Dec 5;10(12):925. doi: 10.1038/s41419-019-2167-z. Cell Death Dis. 2019. PMID: 31804464 Free PMC article.
-
CircHIPK3 regulates the autophagy and apoptosis of hypoxia/reoxygenation-stimulated cardiomyocytes via the miR-20b-5p/ATG7 axis.Cell Death Discov. 2021 Apr 6;7(1):64. doi: 10.1038/s41420-021-00448-6. Cell Death Discov. 2021. PMID: 33824287 Free PMC article.
-
Formononetin may protect aged hearts from ischemia/reperfusion damage by enhancing autophagic degradation.Mol Med Rep. 2018 Dec;18(6):4821-4830. doi: 10.3892/mmr.2018.9544. Epub 2018 Oct 10. Mol Med Rep. 2018. PMID: 30320398 Free PMC article.
References
-
- Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155–2165. - PubMed
-
- Xu A, Szczepanek K, Maceyka MW, Ross T, Bowler E, Hu Y2, Kenny B, Mehfoud C, Desai PN, Baumgarten CM, Chen Q, Lesnefsky EJ. Transient complex I inhibition at the onset of reperfusion by extracellular acidification decreases cardiac injury. Am J Physiol Cell Physiol. 2014;306:C1142–1153. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

