Mass Spectrometry Imaging Reveals Elevated Glomerular ATP/AMP in Diabetes/obesity and Identifies Sphingomyelin as a Possible Mediator
- PMID: 27322466
- PMCID: PMC4909366
- DOI: 10.1016/j.ebiom.2016.03.033
Mass Spectrometry Imaging Reveals Elevated Glomerular ATP/AMP in Diabetes/obesity and Identifies Sphingomyelin as a Possible Mediator
Abstract
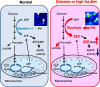
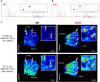
AMP-activated protein kinase (AMPK) is suppressed in diabetes and may be due to a high ATP/AMP ratio, however the quantitation of nucleotides in vivo has been extremely difficult. Via matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI-MSI) to localize renal nucleotides we found that the diabetic kidney had a significant increase in glomerular ATP/AMP ratio. Untargeted MALDI-MSI analysis revealed that a specific sphingomyelin species (SM(d18:1/16:0)) accumulated in the glomeruli of diabetic and high-fat diet-fed mice compared with wild-type controls. In vitro studies in mesangial cells revealed that exogenous addition of SM(d18:1/16:0) significantly elevated ATP via increased glucose consumption and lactate production with a consequent reduction of AMPK and PGC1α. Furthermore, inhibition of sphingomyelin synthases reversed these effects. Our findings suggest that AMPK is reduced in the diabetic kidney due to an increase in the ATP/AMP ratio and that SM(d18:1/16:0) could be responsible for the enhanced ATP production via activation of the glycolytic pathway.
Keywords: AMPK; ATP; Chronic kidney disease; Glycolysis; Matrix-assisted laser desorption/ionization mass spectrometry imaging; Sphingomyelin.
Copyright © 2016 The Authors. Published by Elsevier B.V. All rights reserved.
Figures





References
-
- Baranowski M., Blachnio A., Zabielski P., Gorski J. PPARalpha agonist induces the accumulation of ceramide in the heart of rats fed high-fat diet. J. Physiol. Pharmacol. 2007;58:57–72. - PubMed
-
- Chida J., Kido H. Extraction and quantification of adenosine triphosphate in mammalian tissues and cells. Methods Mol. Biol. 2014;1098:21–32. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

