Climate Change and Aedes Vectors: 21st Century Projections for Dengue Transmission in Europe
- PMID: 27322480
- PMCID: PMC4909611
- DOI: 10.1016/j.ebiom.2016.03.046
Climate Change and Aedes Vectors: 21st Century Projections for Dengue Transmission in Europe
Abstract
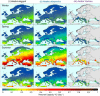
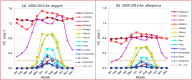
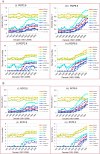
Warming temperatures may increase the geographic spread of vector-borne diseases into temperate areas. Although a tropical mosquito-borne viral disease, a dengue outbreak occurred in Madeira, Portugal, in 2012; the first in Europe since 1920s. This outbreak emphasizes the potential for dengue re-emergence in Europe given changing climates. We present estimates of dengue epidemic potential using vectorial capacity (VC) based on historic and projected temperature (1901-2099). VC indicates the vectors' ability to spread disease among humans. We calculated temperature-dependent VC for Europe, highlighting 10 European cities and three non-European reference cities. Compared with the tropics, Europe shows pronounced seasonality and geographical heterogeneity. Although low, VC during summer is currently sufficient for dengue outbreaks in Southern Europe to commence-if sufficient vector populations (either Ae. aegypti and Ae. albopictus) were active and virus were introduced. Under various climate change scenarios, the seasonal peak and time window for dengue epidemic potential increases during the 21st century. Our study maps dengue epidemic potential in Europe and identifies seasonal time windows when major cities are most conducive for dengue transmission from 1901 to 2099. Our findings illustrate, that besides vector control, mitigating greenhouse gas emissions crucially reduces the future epidemic potential of dengue in Europe.
Keywords: Aedes aegypti; Aedes albopictus; Climate change; Dengue; Temperature; Vectorial capacity.
Copyright © 2016 The Authors. Published by Elsevier B.V. All rights reserved.
Figures


References
-
- Carrieri M., Angelini P., Venturelli C., Maccagnani B., Bellini R. Aedes albopictus (Diptera: Culicidae) population size survey in the 2007 Chikungunya outbreak area in Italy. I. Characterization of breeding sites and evaluation of sampling methodologies. J. Med. Entomol. 2011;48:1214–1225. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

