Safety and Immunogenicity of the Recombinant BCG Vaccine AERAS-422 in Healthy BCG-naïve Adults: A Randomized, Active-controlled, First-in-human Phase 1 Trial
- PMID: 27322481
- PMCID: PMC4909487
- DOI: 10.1016/j.ebiom.2016.04.010
Safety and Immunogenicity of the Recombinant BCG Vaccine AERAS-422 in Healthy BCG-naïve Adults: A Randomized, Active-controlled, First-in-human Phase 1 Trial
Abstract
Background: We report a first-in-human trial evaluating safety and immunogenicity of a recombinant BCG, AERAS-422, over-expressing TB antigens Ag85A, Ag85B, and Rv3407 and expressing mutant perfringolysin.
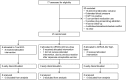
Methods: This was a randomized, double-blind, dose-escalation trial in HIV-negative, healthy adult, BCG-naïve volunteers, negative for prior exposure to Mtb, at one US clinical site. Volunteers were randomized 2:1 at each dose level to receive a single intradermal dose of AERAS-422 (>10(5)-<10(6)CFU=low dose, ≥10(6)-<10(7)CFU=high dose) or non-recombinant Tice BCG (1-8×10(5)CFU). Randomization used an independently prepared randomly generated sequence of treatment assignments. The primary and secondary outcomes were safety and immunogenicity, respectively, assessed in all participants through 182days post-vaccination. ClinicalTrials.gov registration number: NCT01340820.
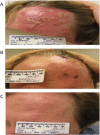
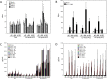
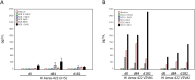
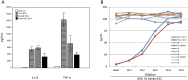
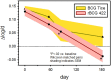
Findings: Between Nov 2010 and Aug 2011, 24 volunteers were enrolled (AERAS-422 high dose, n=8; AERAS-422 low dose, n=8; Tice BCG, n=8); all were included in the safety and immunogenicity analyses. All 24 subjects had at least one adverse event, primarily expected local reactions. High dose AERAS-422 vaccination induced Ag85A- and Ag85B-specific lymphoproliferative responses and marked anti-mycobacterial activity in a whole blood bactericidal activity culture assay (WBA), but was associated with varicella zoster virus (VZV) reactivation in two vaccinees. These volunteers displayed high BCG-specific IFN-γ responses pre- and post-vaccination possibly predisposing them to autocrine/paracrine negative regulation of immune control of latent VZV. A systems biology transcriptomal approach identified positive correlations between post-vaccination T cell expression modules and WBA, and negative correlations between post-vaccination monocyte expression modules and WBA. The expression of one key macrophage marker (F4/80) was constitutively elevated in the two volunteers with zoster.
Interpretation: The unexpected development of VZV in two of eight healthy adult vaccine recipients resulted in discontinuation of AERAS-422 vaccine development. Immunological and transcriptomal data identified correlations with the development of TB immunity and VZV that require further investigation.
Funding: Aeras, FDA, Bill and Melinda Gates Foundation.
Keywords: Functional T cell assays; Herpes zoster;; Recombinant BCG;; Transcriptomes;; Tuberculosis;.
Copyright © 2016 The Authors. Published by Elsevier B.V. All rights reserved.
Figures

Similar articles
-
The safety and immunogenicity of an adenovirus type 35-vectored TB vaccine in HIV-infected, BCG-vaccinated adults with CD4(+) T cell counts >350 cells/mm(3).Vaccine. 2015 Apr 8;33(15):1890-6. doi: 10.1016/j.vaccine.2015.02.004. Epub 2015 Feb 17. Vaccine. 2015. PMID: 25698492 Clinical Trial.
-
Adenovirus type 35-vectored tuberculosis vaccine has an acceptable safety and tolerability profile in healthy, BCG-vaccinated, QuantiFERON(®)-TB Gold (+) Kenyan adults without evidence of tuberculosis.Vaccine. 2016 May 5;34(21):2430-2436. doi: 10.1016/j.vaccine.2016.03.069. Epub 2016 Mar 26. Vaccine. 2016. PMID: 27026148 Clinical Trial.
-
A double-blind, randomised, placebo-controlled, dose-finding trial of the novel tuberculosis vaccine AERAS-402, an adenovirus-vectored fusion protein, in healthy, BCG-vaccinated infants.Vaccine. 2015 Jun 9;33(25):2944-54. doi: 10.1016/j.vaccine.2015.03.070. Epub 2015 Apr 28. Vaccine. 2015. PMID: 25936724 Free PMC article. Clinical Trial.
-
[Novel vaccines against M. tuberculosis].Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese.
-
MVA-85A, a novel candidate booster vaccine for the prevention of tuberculosis in children and adults.Curr Opin Mol Ther. 2010 Feb;12(1):124-34. Curr Opin Mol Ther. 2010. PMID: 20140824 Review.
Cited by
-
Multi-subunit BCG booster vaccine GamTBvac: Assessment of immunogenicity and protective efficacy in murine and guinea pig TB models.PLoS One. 2017 Apr 28;12(4):e0176784. doi: 10.1371/journal.pone.0176784. eCollection 2017. PLoS One. 2017. PMID: 28453555 Free PMC article.
-
Phase 1 Open-Label Dose Escalation Trial for the Development of a Human Bacillus Calmette-Guérin Challenge Model for Assessment of Tuberculosis Immunity In Vivo.J Infect Dis. 2024 May 15;229(5):1498-1508. doi: 10.1093/infdis/jiad441. J Infect Dis. 2024. PMID: 38019956 Free PMC article. Clinical Trial.
-
Fighting Tuberculosis: In Search of a BCG Replacement.Microorganisms. 2022 Dec 23;11(1):51. doi: 10.3390/microorganisms11010051. Microorganisms. 2022. PMID: 36677343 Free PMC article. Review.
-
Recombinant BCG to Enhance Its Immunomodulatory Activities.Vaccines (Basel). 2022 May 23;10(5):827. doi: 10.3390/vaccines10050827. Vaccines (Basel). 2022. PMID: 35632582 Free PMC article. Review.
-
Bridging the gaps to overcome major hurdles in the development of next-generation tuberculosis vaccines.Front Immunol. 2023 Aug 11;14:1193058. doi: 10.3389/fimmu.2023.1193058. eCollection 2023. Front Immunol. 2023. PMID: 37638056 Free PMC article. Review.
References
-
- Horwitz M.A., Harth G., Dillon B.J., Maslesa-Galic S. Recombinant bacillus calmette-guerin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc. Natl. Acad. Sci. U S A. 2000;97:13853–13858. - PMC - PubMed
-
- Sun R., Skeiky Y.A., Izzo A. Novel recombinant BCG expressing perfringolysin O and the over-expression of key immunodominant antigens; pre-clinical characterization, safety and protection against challenge with Mycobacterium tuberculosis. Vaccine. 2009;27:4412–4423. - PubMed
-
- Hoft D.F., Worku S., Kampmann B. Investigation of the relationships between immune-mediated inhibition of mycobacterial growth and other potential surrogate markers of protective Mycobacterium tuberculosis immunity. J. Infect. Dis. 2002;186:1448–1457. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

