Regulation of Epithelial-to-Mesenchymal Transition Using Biomimetic Fibrous Scaffolds
- PMID: 27322677
- PMCID: PMC5070665
- DOI: 10.1021/acsami.6b05646
Regulation of Epithelial-to-Mesenchymal Transition Using Biomimetic Fibrous Scaffolds
Abstract
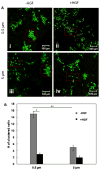
Epithelial-to-mesenchymal transition (EMT) is a well-studied biological process that takes place during embryogenesis, carcinogenesis, and tissue fibrosis. During EMT, the polarized epithelial cells with a cuboidal architecture adopt an elongated fibroblast-like morphology. This process is accompanied by the expression of many EMT-specific molecular markers. Although the molecular mechanism leading to EMT has been well-established, the effects of matrix topography and microstructure have not been clearly elucidated. Synthetic scaffolds mimicking the meshlike structure of the basement membrane with an average fiber diameter of 0.5 and 5 μm were produced via the electrospinning of poly(ε-caprolactone) (PCL) and were used to test the significance of fiber diameter on EMT. Cell-adhesive peptide motifs were conjugated to the fiber surface to facilitate cell attachment. Madin-Darby Canine Kidney (MDCK) cells grown on these substrates showed distinct phenotypes. On 0.5 μm substrates, cells grew as compact colonies with an epithelial phenotype. On 5 μm scaffolds, cells were more individually dispersed and appeared more fibroblastic. Upon the addition of hepatocyte growth factor (HGF), an EMT inducer, cells grown on the 0.5 μm scaffold underwent pronounced scattering, as evidenced by the alteration of cell morphology, localization of focal adhesion complex, weakening of cell-cell adhesion, and up-regulation of mesenchymal markers. In contrast, HGF did not induce a pronounced scattering of MDCK cells cultured on the 5.0 μm scaffold. Collectively, our results show that the alteration of the fiber diameter of proteins found in the basement membrane may create enough disturbances in epithelial organization and scattering that might have important implications in disease progression.
Keywords: MDCK cells; epithelial-to-mesenchymal transition; fiber diameter; fibrous scaffolds; hepatocyte growth factor; phenotype.
Figures








References
-
- Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–3161. - PubMed
-
- Moreno-Bueno G, Portillo F, Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene. 2008;27:6958–6969. - PubMed
-
- Peinado H, Olmeda D, Cano A, Snail Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nature Reviews Cancer. 2007;7:415–428. - PubMed
-
- Thiery JP, Sleeman JP. Complex networks orchestrate epithelial–mesenchymal transitions. Nature reviews Molecular cell biology. 2006;7:131–142. - PubMed
-
- Nakaya Y, Sukowati EW, Wu Y, Sheng G. RhoA and microtubule dynamics control cell–basement membrane interaction in EMT during gastrulation. Nature cell biology. 2008;10:765–775. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

