The anti-apoptotic PON2 protein is Wnt/β-catenin-regulated and correlates with radiotherapy resistance in OSCC patients
- PMID: 27322774
- PMCID: PMC5239460
- DOI: 10.18632/oncotarget.9013
The anti-apoptotic PON2 protein is Wnt/β-catenin-regulated and correlates with radiotherapy resistance in OSCC patients
Abstract
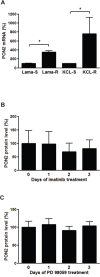
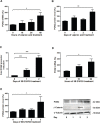
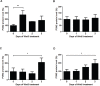
Aberrant Wnt signaling and control of anti-apoptotic mechanisms are pivotal features in different types of cancer to undergo cell death programs. The intracellular human enzyme Paraoxonase-2 (PON2) is known to have anti-apoptotic properties in leukemia and oral squamous cell cancer (OSCC) cells. However, the distinct regulating pathways are poorly understood. First, we present a so far unknown regulation of PON2 protein expression through the Wnt/GSK3β/β-catenin pathway in leukemia and OSCC cells. This was confirmed via in silico analysis, promoter reporter studies and treatment of multiple cell lines (K562, SCC-4, PCI-13) with different Wnt ligands/inhibitors in vitro. Ex vivo analysis of OSCC patients revealed a correlation between PON2 and β-catenin expression in tumor tissue. Higher PON2 expression in OSCC is associated with relapse independently of treatment (e.g. surgery/radio-/chemotherapy). These results emphasize the clinical impact of the newly described regulation of PON2 through Wnt/GSK3β/β-catenin. More importantly, the study revealed the fundamental finding of an overall Wnt/GSK3β/β-catenin dependent regulation of PON2 in different cancers, which was confirmed by systematic and multimethodological approaches. Thus, the herein presented mechanistic insight contributes to a better understanding of tumor specific escape from cell death strategies and suggests PON2 as a new potential biomarker for therapy resistance or as a prognostic tumor marker.
Keywords: Wnt / beta-catenin; leukemia; oral squamous cancer cell; paraoxonase-2; tumor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

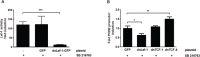


References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. - PubMed
-
- Logan CY, Nusse R. The wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. - PubMed
-
- Morgan RG, Ridsdale J, Tonks A, Darley RL. Factors affecting the nuclear localization of beta-catenin in normal and malignant tissue. J Cell Biochem. 2014;115:1351–1361. - PubMed
-
- Staal FJ, Clevers HC. Wnt signalling and haematopoiesis: A wnt-wnt situation. Nat Rev Immunol. 2005;5:21–30. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

