GPR142 Controls Tryptophan-Induced Insulin and Incretin Hormone Secretion to Improve Glucose Metabolism
- PMID: 27322810
- PMCID: PMC4920590
- DOI: 10.1371/journal.pone.0157298
GPR142 Controls Tryptophan-Induced Insulin and Incretin Hormone Secretion to Improve Glucose Metabolism
Abstract
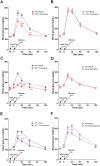
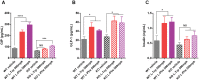
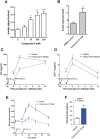
GPR142, a putative amino acid receptor, is expressed in pancreatic islets and the gastrointestinal tract, but the ligand affinity and physiological role of this receptor remain obscure. In this study, we show that in addition to L-Tryptophan, GPR142 signaling is also activated by L-Phenylalanine but not by other naturally occurring amino acids. Furthermore, we show that Tryptophan and a synthetic GPR142 agonist increase insulin and incretin hormones and improve glucose disposal in mice in a GPR142-dependent manner. In contrast, Phenylalanine improves in vivo glucose disposal independently of GPR142. Noteworthy, refeeding-induced elevations in insulin and glucose-dependent insulinotropic polypeptide are blunted in Gpr142 null mice. In conclusion, these findings demonstrate GPR142 is a Tryptophan receptor critically required for insulin and incretin hormone regulation and suggest GPR142 agonists may be effective therapies that leverage amino acid sensing pathways for the treatment of type 2 diabetes.
Conflict of interest statement
Figures


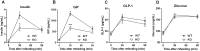
Similar articles
-
The aromatic amino acid sensor GPR142 controls metabolism through balanced regulation of pancreatic and gut hormones.Mol Metab. 2019 Jan;19:49-64. doi: 10.1016/j.molmet.2018.10.012. Epub 2018 Nov 5. Mol Metab. 2019. PMID: 30472415 Free PMC article.
-
GPR142 Agonists Stimulate Glucose-Dependent Insulin Secretion via Gq-Dependent Signaling.PLoS One. 2016 Apr 22;11(4):e0154452. doi: 10.1371/journal.pone.0154452. eCollection 2016. PLoS One. 2016. PMID: 27104960 Free PMC article.
-
Differential role of GPR142 in tryptophan-mediated enhancement of insulin secretion in obese and lean mice.PLoS One. 2018 Jun 11;13(6):e0198762. doi: 10.1371/journal.pone.0198762. eCollection 2018. PLoS One. 2018. PMID: 29889885 Free PMC article.
-
Potential New Approaches to Modifying Intestinal GLP-1 Secretion in Patients with Type 2 Diabetes Mellitus : Focus on Bile Acid Sequestrants.Clin Drug Investig. 2012 Jan;32(1):1-14. doi: 10.2165/11595370-000000000-00000. Clin Drug Investig. 2012. PMID: 27933595 Review.
-
β-Cell glutamate signaling: Its role in incretin-induced insulin secretion.J Diabetes Investig. 2016 Apr;7 Suppl 1(Suppl 1):38-43. doi: 10.1111/jdi.12468. Epub 2016 Mar 14. J Diabetes Investig. 2016. PMID: 27186354 Free PMC article. Review.
Cited by
-
Nutrient-Induced Cellular Mechanisms of Gut Hormone Secretion.Nutrients. 2021 Mar 9;13(3):883. doi: 10.3390/nu13030883. Nutrients. 2021. PMID: 33803183 Free PMC article. Review.
-
Revisiting the concept of incretin and enteroendocrine L-cells as type 2 diabetes mellitus treatment.Pharmacol Res. 2022 Jun;180:106237. doi: 10.1016/j.phrs.2022.106237. Epub 2022 Apr 26. Pharmacol Res. 2022. PMID: 35487405 Free PMC article. Review.
-
Assessment of the dietary amino acid profiles and the relative biomarkers for amino acid balance in the low-protein diets for broiler chickens.J Anim Sci Biotechnol. 2024 Nov 14;15(1):157. doi: 10.1186/s40104-024-01108-2. J Anim Sci Biotechnol. 2024. PMID: 39538238 Free PMC article.
-
Amino Acid Catabolism: An Overlooked Area of Metabolism.Nutrients. 2023 Jul 29;15(15):3378. doi: 10.3390/nu15153378. Nutrients. 2023. PMID: 37571315 Free PMC article. Review.
-
Pharmacology and function of the orphan GPR139 G protein-coupled receptor.Basic Clin Pharmacol Toxicol. 2020 Jun;126 Suppl 6(Suppl 6):35-46. doi: 10.1111/bcpt.13263. Epub 2019 Jun 27. Basic Clin Pharmacol Toxicol. 2020. PMID: 31132229 Free PMC article. Review.
References
-
- Tsujihata Y, Ito R, Suzuki M, Harada A, Negoro N, Yasuma T, et al. TAK-875, an orally available G protein-coupled receptor 40/free fatty acid receptor 1 agonist, enhances glucose-dependent insulin secretion and improves both postprandial and fasting hyperglycemia in type 2 diabetic rats. The Journal of pharmacology and experimental therapeutics. 2011;339(1):228–37. 10.1124/jpet.111.183772 . - DOI - PubMed
-
- Kaku K, Enya K, Nakaya R, Ohira T, Matsuno R. Efficacy and safety of fasiglifam (TAK-875), a G protein-coupled receptor 40 agonist, in Japanese patients with type 2 diabetes inadequately controlled by diet and exercise: a randomized, double-blind, placebo-controlled, phase III trial. Diabetes, obesity & metabolism. 2015;17(7):675–81. 10.1111/dom.12467 . - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

