Tunable, biodegradable gold nanoparticles as contrast agents for computed tomography and photoacoustic imaging
- PMID: 27322961
- PMCID: PMC4941627
- DOI: 10.1016/j.biomaterials.2016.06.015
Tunable, biodegradable gold nanoparticles as contrast agents for computed tomography and photoacoustic imaging
Abstract
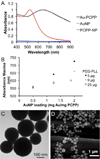
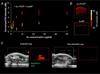
Gold nanoparticles (AuNP) have been proposed for many applications in medicine. Although large AuNP (>5.5 nm) are desirable for their longer blood circulation and accumulation in diseased tissues, small AuNP (<5.5 nm) are required for excretion via the kidneys. We present a novel platform where small, excretable AuNP are encapsulated into biodegradable poly di(carboxylatophenoxy)phosphazene (PCPP) nanospheres. These larger nanoparticles (Au-PCPP) can perform their function as contrast agents, then subsequently break down into harmless byproducts and release the AuNP for swift excretion. Homogeneous Au-PCPP were synthesized using a microfluidic device. The size of the Au-PCPP can be controlled by the amount of polyethylene glycol-polylysine (PEG-PLL) block co-polymer in the formulation. Synthesis of Au-PCPP nanoparticles and encapsulation of AuNP in PCPP were evaluated using transmission electron microscopy and their biocompatibility and biodegradability confirmed in vitro. The Au-PCPP nanoparticles were found to produce strong computed tomography contrast. The UV-Vis absorption peak of Au-PCPP can be tuned into the near infrared region via inclusion of varying amounts of AuNP and controlling the nanoparticle size. In vitro and in vivo experiments demonstrated the potential of Au-PCPP as contrast agents for photoacoustic imaging. Therefore, Au-PCPP nanoparticles have high potency as contrast agents for two imaging modalities, as well as being biocompatible and biodegradable, and thus represent a platform with potential for translation into the clinic.
Keywords: Computed tomography; Contrast agent; Gold nanoparticle; Metal-polymer nanoparticle; Photoacoustic imaging.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures



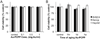


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

