PTP1B controls non-mitochondrial oxygen consumption by regulating RNF213 to promote tumour survival during hypoxia
- PMID: 27323329
- PMCID: PMC4936519
- DOI: 10.1038/ncb3376
PTP1B controls non-mitochondrial oxygen consumption by regulating RNF213 to promote tumour survival during hypoxia
Abstract
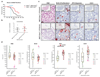
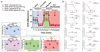
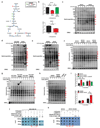
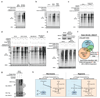
Tumours exist in a hypoxic microenvironment and must limit excessive oxygen consumption. Hypoxia-inducible factor (HIF) controls mitochondrial oxygen consumption, but how/if tumours regulate non-mitochondrial oxygen consumption (NMOC) is unknown. Protein-tyrosine phosphatase-1B (PTP1B) is required for Her2/Neu-driven breast cancer (BC) in mice, although the underlying mechanism and human relevance remain unclear. We found that PTP1B-deficient HER2(+) xenografts have increased hypoxia, necrosis and impaired growth. In vitro, PTP1B deficiency sensitizes HER2(+) BC lines to hypoxia by increasing NMOC by α-KG-dependent dioxygenases (α-KGDDs). The moyamoya disease gene product RNF213, an E3 ligase, is negatively regulated by PTP1B in HER2(+) BC cells. RNF213 knockdown reverses the effects of PTP1B deficiency on α-KGDDs, NMOC and hypoxia-induced death of HER2(+) BC cells, and partially restores tumorigenicity. We conclude that PTP1B acts via RNF213 to suppress α-KGDD activity and NMOC. This PTP1B/RNF213/α-KGDD pathway is critical for survival of HER2(+) BC, and possibly other malignancies, in the hypoxic tumour microenvironment.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

