Single, immediate postoperative instillation of chemotherapy in non-muscle invasive bladder cancer: a systematic review and network meta-analysis of randomized clinical trials using different drugs
- PMID: 27323781
- PMCID: PMC5216735
- DOI: 10.18632/oncotarget.9991
Single, immediate postoperative instillation of chemotherapy in non-muscle invasive bladder cancer: a systematic review and network meta-analysis of randomized clinical trials using different drugs
Abstract
We performed a network meta-analysis of randomized controlled trials (RCTs) to compare the efficacy of several intravesical chemotherapeutic (IVC) agents after transurethral resection of bladder tumor (TURB) in non-muscle invasive bladder cancer patients. The literature search was conducted using the Embase, Scopus and PubMed databases for RCTs, including patients with single or multiple, primary or recurrent stage Ta or T1 urothelial carcinoma of the bladder managed with a single, immediate instillation of IVC after TURB. Thirteen RCTs met the eligibility criteria. Pair-wise meta-analysis (direct comparison) showed that pirarubicin [hazard ratio (HR): 0.31], epirubicin (HR: 0.62), and MMC (HR: 0.40) were the most effective drugs for reducing tumor recurrence. Bayesian network meta-analysis (indirect comparison) revealed that treatment with pirarubicin (HR: 0.31), MMC (HR: 0.44), or epirubicin (HR: 0.60) was associated with prolonged recurrence-free survival. Among the drugs examined, only pirarubicin reduced disease progression compared to controls. These results suggest that a single, immediate administration of IVC with pirarubicin, MMC, or epirubicin is associated with prolonged recurrence-free survival following TURB in non-muscle invasive bladder cancer patients, though only pirarubicin also reduced disease progression.
Keywords: chemotherapy; drug therapy; single instillation; systematic review; urinary bladder neoplasm.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures

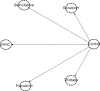
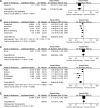


References
-
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. - PubMed
-
- Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Comperat E, Sylvester RJ, Kaasinen E, Bohle A, Palou Redorta J, Rouprêt M, European Association of Urology EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013;64:639–653. - PubMed
-
- Hall MC, Chang SS, Dalbagni G, Pruthi RS, Seigne JD, Skinner EC, Wolf JS, Jr, Schellhammer PF. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol. 2007;178:2314–2330. - PubMed
-
- Cookson MS, Chang SS, Oefelein MG, Gallagher JR, Schwartz B, Heap K. National practice patterns for immediate postoperative instillation of chemotherapy in nonmuscle invasive bladder cancer. J Urol. 2012;187:1571–1576. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

