Treg engage lymphotoxin beta receptor for afferent lymphatic transendothelial migration
- PMID: 27323847
- PMCID: PMC4919545
- DOI: 10.1038/ncomms12021
Treg engage lymphotoxin beta receptor for afferent lymphatic transendothelial migration
Abstract
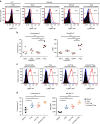
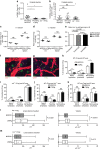
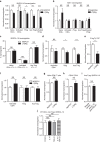
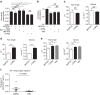
Regulatory T cells (Tregs) are essential to suppress unwanted immunity or inflammation. After islet allo-transplant Tregs must migrate from blood to allograft, then via afferent lymphatics to draining LN to protect allografts. Here we show that Tregs but not non-Treg T cells use lymphotoxin (LT) during migration from allograft to draining LN, and that LT deficiency or blockade prevents normal migration and allograft protection. Treg LTαβ rapidly modulates cytoskeletal and membrane structure of lymphatic endothelial cells; dependent on VCAM-1 and non-canonical NFκB signalling via LTβR. These results demonstrate a form of T-cell migration used only by Treg in tissues that serves an important role in their suppressive function and is a unique therapeutic focus for modulating suppression.
Figures

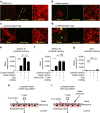
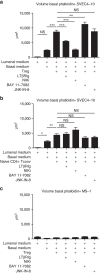

References
-
- Ochando J. C. et al.. Lymph node occupancy is required for the peripheral development of alloantigen-specific Foxp3+ regulatory T cells. J. Immunol. 174, 6993–7005 (2005). - PubMed
-
- Bai Y. et al.. L-selectin-dependent lymphoid occupancy is required to induce alloantigen-specific tolerance. J. Immunol. 168, 1579–1589 (2002). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

