Enhanced Ghrelin Levels and Hypothalamic Orexigenic AgRP and NPY Neuropeptide Expression in Models of Jejuno-Colonic Short Bowel Syndrome
- PMID: 27323884
- PMCID: PMC4914859
- DOI: 10.1038/srep28345
Enhanced Ghrelin Levels and Hypothalamic Orexigenic AgRP and NPY Neuropeptide Expression in Models of Jejuno-Colonic Short Bowel Syndrome
Abstract
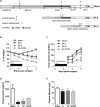
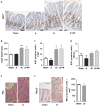
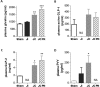
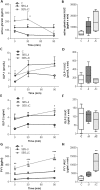
Short bowel syndrome (SBS) patients developing hyperphagia have a better outcome. Gastrointestinal endocrine adaptations help to improve intestinal functions and food behaviour. We investigated neuroendocrine adaptations in SBS patients and rat models with jejuno-ileal (IR-JI) or jejuno-colonic (IR-JC) anastomosis with and without parenteral nutrition. Circulating levels of ghrelin, PYY, GLP-1, and GLP-2 were determined in SBS rat models and patients. Levels of mRNA for proglucagon, PYY and for hypothalamic neuropeptides were quantified by qRT-PCR in SBS rat models. Histology and immunostaining for Ki67, GLP-1 and PYY were performed in SBS rats. IR-JC rats, but not IR-JI, exhibited significantly higher crypt depths and number of Ki67-positive cells than sham. Fasting and/or postprandial plasma ghrelin and PYY concentrations were higher, or tend to be higher, in IR-JC rats and SBS-JC patients than in controls. Proglucagon and Pyy mRNA levels were significantly enhanced in IR-JC rats. Levels of mRNA coding hypothalamic orexigenic NPY and AgRP peptides were significantly higher in IR-JC than in sham rats. We demonstrate an increase of plasma ghrelin concentrations, major changes in hypothalamic neuropeptides levels and greater induction of PYY in SBS-JC rats and patients suggesting that jejuno-colonic continuity creates a peculiar environment promoting further gut-brain adaptations.
Figures


References
-
- Nordgaard I., Hansen B. S. & Mortensen P. B. Importance of colonic support for energy absorption as small-bowel failure proceeds. Am. J. Clin. Nutr. 64, 222–231 (1996). - PubMed
-
- Messing B. et al.. Intestinal absorption of free oral hyperalimentation in the very short bowel syndrome. Gastroenterology 100, 1502–1508 (1991). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

