Anti-obesity effect of intranasal administration of galanin-like peptide (GALP) in obese mice
- PMID: 27323911
- PMCID: PMC4914964
- DOI: 10.1038/srep28200
Anti-obesity effect of intranasal administration of galanin-like peptide (GALP) in obese mice
Abstract
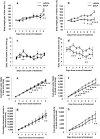
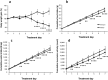
Galanin-like peptide (GALP) has an anti-obesity effect in rats and mice. It has been reported that the uptake of GALP by the brain is higher after intranasal administration than with intravenous injection. This study therefore aimed to clarify the effect of intranasal administration of GALP on the feeding behavior of lean and obese mice. Autoradiography revealed the presence of (125)I-GALP in the olfactory bulb and the brain microcirculation. The body weights of ob/ob mice gradually increased during vehicle treatment, but remained unchanged in response to repeated intranasal administration of GALP, with both ob/ob and diet-induced obese mice displaying significantly decreased food intake, water intake and locomotor activity when treated with GALP. These results suggest that intranasal administration is an effective route whereby GALP can exert its effect as an anti-obesity drug.
Figures





Similar articles
-
Transcriptomic (DNA Microarray) and Metabolome (LC-TOF-MS) Analyses of the Liver in High-Fat Diet Mice after Intranasal Administration of GALP (Galanin-like Peptide).Int J Mol Sci. 2023 Oct 31;24(21):15825. doi: 10.3390/ijms242115825. Int J Mol Sci. 2023. PMID: 37958806 Free PMC article.
-
Regulation of Feeding Behavior and Energy Metabolism by Galanin-like Peptide (GALP): A Novel Strategy to Fight Against Obesity.Curr Pharm Des. 2018;24(33):3926-3933. doi: 10.2174/1381612824666181106111623. Curr Pharm Des. 2018. PMID: 30398112 Review.
-
Effect of Intranasal Administration of Galanin-like Peptide (GALP) on Body Weight and Hepatic Lipids Accumulation in Mice with Diet-induced Obesity.Curr Pharm Des. 2017;23(25):3751-3756. doi: 10.2174/1381612823666170321095950. Curr Pharm Des. 2017. PMID: 28325141
-
Exaggerated feeding response to central galanin-like peptide administration in diet-induced obese rats.Neuropeptides. 2005 Jun;39(3):333-6. doi: 10.1016/j.npep.2004.12.025. Epub 2005 Feb 16. Neuropeptides. 2005. PMID: 15944031
-
Galanin-like peptide: a key player in the homeostatic regulation of feeding and energy metabolism?Int J Obes (Lond). 2011 May;35(5):619-28. doi: 10.1038/ijo.2010.202. Epub 2010 Oct 12. Int J Obes (Lond). 2011. PMID: 20938442 Review.
Cited by
-
Effect of Intracerebroventricular Administration of Galanin-Like Peptide on Hepatokines in C57BL/6 J Mice.J Mol Neurosci. 2024 Feb 22;74(1):25. doi: 10.1007/s12031-024-02200-y. J Mol Neurosci. 2024. PMID: 38386221
-
Neuropeptidergic Control of Feeding: Focus on the Galanin Family of Peptides.Int J Mol Sci. 2021 Mar 3;22(5):2544. doi: 10.3390/ijms22052544. Int J Mol Sci. 2021. PMID: 33802616 Free PMC article. Review.
-
Intranasal delivery of a Fas-blocking peptide attenuates Fas-mediated apoptosis in brain ischemia.Sci Rep. 2018 Oct 9;8(1):15041. doi: 10.1038/s41598-018-33296-z. Sci Rep. 2018. PMID: 30301943 Free PMC article.
-
Galanin Protects from Caspase-8/12-initiated Neuronal Apoptosis in the Ischemic Mouse Brain via GalR1.Aging Dis. 2017 Feb 1;8(1):85-100. doi: 10.14336/AD.2016.0806. eCollection 2017 Feb. Aging Dis. 2017. PMID: 28203483 Free PMC article.
-
Transcriptomic (DNA Microarray) and Metabolome (LC-TOF-MS) Analyses of the Liver in High-Fat Diet Mice after Intranasal Administration of GALP (Galanin-like Peptide).Int J Mol Sci. 2023 Oct 31;24(21):15825. doi: 10.3390/ijms242115825. Int J Mol Sci. 2023. PMID: 37958806 Free PMC article.
References
-
- Ohtaki T. et al.. Isolation and cDNA cloning of a novel galanin-like peptide (GALP) from porcine hypothalamus. J Biol Chem 274, 37041–37045 (1999). - PubMed
-
- Krasnow S. M., Hohmann J. G., Gragerov A., Clifton D. K. & Steiner R. A. Analysis of the contribution of galanin receptors 1 and 2 to the central actions of galanin-like peptide. Neuroendocrinology 79, 268–277 (2004). - PubMed
-
- Juréus A., Cunningham M. J., McClain M. E., Clifton D. K. & Steiner R. A. Galanin-like peptide (GALP) is a target for regulation by leptin in the hypothalamus of the rat. Endocrinology 141, 2703–2706 (2000). - PubMed
-
- Juréus A. et al.. Distribution and regulation of galanin-like peptide (GALP) in the hypothalamus of the mouse. Endocrinology 142, 5140–5144 (2001). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

