Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration
- PMID: 27324127
- PMCID: PMC5010608
- DOI: 10.1007/s00441-016-2431-9
Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration
Abstract
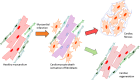
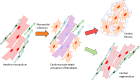
Ischemic cell death during a myocardial infarction leads to a multiphase reparative response in which the damaged tissue is replaced with a fibrotic scar produced by fibroblasts and myofibroblasts. This also induces geometrical, biomechanical, and biochemical changes in the uninjured ventricular wall eliciting a reactive remodeling process that includes interstitial and perivascular fibrosis. Although the initial reparative fibrosis is crucial for preventing rupture of the ventricular wall, an exaggerated fibrotic response and reactive fibrosis outside the injured area are detrimental as they lead to progressive impairment of cardiac function and eventually to heart failure. In this review, we summarize current knowledge of the mechanisms of both reparative and reactive cardiac fibrosis in response to myocardial infarction, discuss the potential of inducing cardiac regeneration through direct reprogramming of fibroblasts and myofibroblasts into cardiomyocytes, and review the currently available and potential future therapeutic strategies to inhibit cardiac fibrosis. Graphical abstract Reparative response following a myocardial infarction. Hypoxia-induced cardiomyocyte death leads to the activation of myofibroblasts and a reparative fibrotic response in the injured area. Right top In adult mammals, the fibrotic scar formed at the infarcted area is permanent and promotes reactive fibrosis in the uninjured myocardium. Right bottom In teleost fish and newts and in embryonic and neonatal mammals, the initial formation of a fibrotic scar is followed by regeneration of the cardiac muscle tissue. Induction of post-infarction cardiac regeneration in adult mammals is currently the target of intensive research and drug discovery attempts.
Keywords: Anti-fibrotic therapy; Cardiac fibrosis; Cardiac regeneration; Myocardial infarction; Pro-fibrotic signaling.
Conflict of interest statement
The authors declare that they have no conflict of interest. Research involving human participants and/or animals This article does not contain any studies performed by any of the authors on human participants or animals.
Figures

References
-
- Addis RC, Ifkovits JL, Pinto F, Kellam LD, Esteso P, Rentschler S, Christoforou N, Epstein JA, Gearhart JD. Optimization of direct fibroblast reprogramming to cardiomyocytes using calcium activity as a functional measure of success. J Mol Cell Cardiol. 2013;60:97–106. doi: 10.1016/j.yjmcc.2013.04.004. - DOI - PMC - PubMed
-
- Ali SR, Ranjbarvaziri S, Talkhabi M, Zhao P, Subat A, Hojjat A, Kamran P, Muller AM, Volz KS, Tang Z, Red-Horse K, Ardehali R. Developmental heterogeneity of cardiac fibroblasts does not predict pathological proliferation and activation. Circ Res. 2014;115:625–635. doi: 10.1161/CIRCRESAHA.115.303794. - DOI - PubMed
-
- Arslan F, Smeets MB, Riem Vis PW, Karper JC, Quax PH, Bongartz LG, Peters JH, Hoefer IE, Doevendans PA, Pasterkamp G, de Kleijn DP. Lack of fibronectin-EDA promotes survival and prevents adverse remodeling and heart function deterioration after myocardial infarction. Circ Res. 2011;108:582–592. doi: 10.1161/CIRCRESAHA.110.224428. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

