Catestatin Gly364Ser Variant Alters Systemic Blood Pressure and the Risk for Hypertension in Human Populations via Endothelial Nitric Oxide Pathway
- PMID: 27324226
- PMCID: PMC4945419
- DOI: 10.1161/HYPERTENSIONAHA.116.06568
Catestatin Gly364Ser Variant Alters Systemic Blood Pressure and the Risk for Hypertension in Human Populations via Endothelial Nitric Oxide Pathway
Abstract
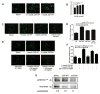
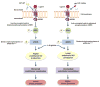
Catestatin (CST), an endogenous antihypertensive/antiadrenergic peptide, is a novel regulator of cardiovascular physiology. Here, we report case-control studies in 2 geographically/ethnically distinct Indian populations (n≈4000) that showed association of the naturally-occurring human CST-Gly364Ser variant with increased risk for hypertension (age-adjusted odds ratios: 1.483; P=0.009 and 2.951; P=0.005). Consistently, 364Ser allele carriers displayed elevated systolic (up to ≈8 mm Hg; P=0.004) and diastolic (up to ≈6 mm Hg; P=0.001) blood pressure. The variant allele was also found to be in linkage disequilibrium with other functional single-nucleotide polymorphisms in the CHGA promoter and nearby coding region. Functional characterization of the Gly364Ser variant was performed using cellular/molecular biological experiments (viz peptide-receptor binding assays, nitric oxide [NO], phosphorylated extracellular regulated kinase, and phosphorylated endothelial NO synthase estimations) and computational approaches (molecular dynamics simulations for structural analysis of wild-type [CST-WT] and variant [CST-364Ser] peptides and docking of peptide/ligand with β-adrenergic receptors [ADRB1/2]). CST-WT and CST-364Ser peptides differed profoundly in their secondary structures and showed differential interactions with ADRB2; although CST-WT displaced the ligand bound to ADRB2, CST-364Ser failed to do the same. Furthermore, CST-WT significantly inhibited ADRB2-stimulated extracellular regulated kinase activation, suggesting an antagonistic role towards ADRB2 unlike CST-364Ser. Consequently, CST-WT was more potent in NO production in human umbilical vein endothelial cells as compared with CST-364Ser. This NO-producing ability of CST-WT was abrogated by ADRB2 antagonist ICI 118551. In conclusion, CST-364Ser allele enhanced the risk for hypertension in human populations, possibly via diminished endothelial NO production because of altered interactions of CST-364Ser peptide with ADRB2 as compared with CST-WT.
Keywords: chromogranin A; genetic association study; genetic variation; hypertension; nitric oxide.
© 2016 American Heart Association, Inc.
Figures

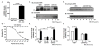
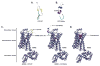

References
-
- O’Connor DT, Takiyyuddin MA, Printz MP, Dinh TQ, Barbosa JA, Rozansky DJ, Mahata SK, Wu H, Kennedy BP, Ziegler MG, Wright FA, Schlager G, Parmer RJ. Catecholamine storage vesicle protein expression in genetic hypertension. Blood Press. 1999;8:285–295. - PubMed
-
- Takiyyuddin MA, De Nicola L, Gabbai FB, Dinh TQ, Kennedy B, Ziegler MG, Sabban EL, Parmer RJ, O’Connor DT. Catecholamine secretory vesicles. Augmented chromogranins and amines in secondary hypertension. Hypertension. 1993;21:674–679. - PubMed
-
- Estensen ME, Hognestad A, Syversen U, Squire I, Ng L, Kjekshus J, Dickstein K, Omland T. Prognostic value of plasma chromogranin a levels in patients with complicated myocardial infarction. Am Heart J. 2006;152:e1–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

