Treatment with rhDNase in patients with cystic fibrosis alters in-vitro CHIT-1 activity of isolated leucocytes
- PMID: 27324468
- PMCID: PMC4991521
- DOI: 10.1111/cei.12827
Treatment with rhDNase in patients with cystic fibrosis alters in-vitro CHIT-1 activity of isolated leucocytes
Abstract
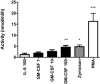
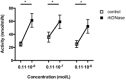
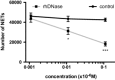
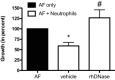
Recent data suggest a possible relationship between cystic fibrosis (CF) pharmacotherapy, Aspergillus fumigatus colonization (AC) and/or allergic bronchopulmonary aspergillosis (ABPA). The aim of this study was to determine if anti-fungal defence mechanisms are influenced by CF pharmacotherapy, i.e. if (1) neutrophils form CF and non-CF donors differ in their ability to produce chitotriosidase (CHIT-1); (2) if incubation of isolated neutrophils with azithromycin, salbutamol, prednisolone or rhDNase might influence the CHIT-1 activity; and (3) if NETosis and neutrophil killing efficiency is influenced by rhDNase. Neutrophils were isolated from the blood of CF patients (n = 19; mean age 26·8 years or healthy, non-CF donors (n = 20; 38·7 years) and stimulated with phorbol-12-myristate-13-acetate (PMA), azithromycin, salbutamol, prednisolone or rhDNase. CHIT-1 enzyme activity was measured with a fluorescent substrate. NETosis was induced by PMA and neutrophil killing efficiency was assessed by a hyphae recovery assay. Neutrophil CHIT-1 activity was comparable in the presence or absence of PMA stimulation in both CF and non-CF donors. PMA stimulation and preincubation with rhDNase increased CHIT-1 activity in culture supernatants from non-CF and CF donors. However, this increase was significant in non-CF donors but not in CF patients (P < 0·05). RhDNase reduced the number of NETs in PMA-stimulated neutrophils and decreased the killing efficiency of leucocytes in our in-vitro model. Azithromycin, salbutamol or prednisolone had no effect on CHIT-1 activity. Stimulation of isolated leucocytes with PMA and treatment with rhDNase interfered with anti-fungal defence mechanisms. However, the impact of our findings for treatment in CF patients needs to be proved in a clinical cohort.
Keywords: fungal; human; inflammation; lung; neutrophils.
© 2016 British Society for Immunology.
Figures


References
-
- Hedayati MT, Mayahi S, Denning DW. A study on Aspergillus species in houses of asthmatic patients from Sari City, Iran and a brief review of the health effects of exposure to indoor Aspergillus. Environ Monit Assess 2010; 168:481–7. - PubMed
-
- Stevens DA, Moss RB, Kurup VP et al Allergic bronchopulmonary aspergillosis in cystic fibrosis–state of the art: Cystic Fibrosis Foundation Consensus Conference. Clin Infect Dis 2003; 37 (Suppl. 3):S225–64. - PubMed
-
- Gazendam RP, van Hamme JL, Tool AT et al Human neutrophils use different mechanisms to kill Aspergillus fumigatus conidia and hyphae: evidence from phagocyte defects. J Immunol 2016; 196:1272–83. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

