Predictors of Inadequate Linezolid Concentrations after Standard Dosing in Critically Ill Patients
- PMID: 27324768
- PMCID: PMC4997846
- DOI: 10.1128/AAC.00356-16
Predictors of Inadequate Linezolid Concentrations after Standard Dosing in Critically Ill Patients
Abstract
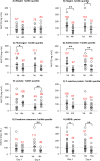
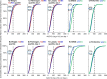
Adequate linezolid blood concentrations have been shown to be associated with an improved clinical outcome. Our goal was to assess new predictors of inadequate linezolid concentrations often observed in critically ill patients. Fifty-two critically ill patients with severe infections receiving standard dosing of linezolid participated in this prospective observational study. Serum samples (median, 32 per patient) were taken on four consecutive days, and total linezolid concentrations were quantified. Covariates influencing linezolid pharmacokinetics were identified by multivariate analysis and a population pharmacokinetic model. Target attainment (area under the concentration-time curve over 12 h [AUC12]/MIC ratio of >50; MIC = 2 mg/liter) was calculated for both the study patients and a simulated independent patient group (n = 67,000). Target attainment was observed for only 36% of the population on both days 1 and 4. Independent covariates related to significant decreases of linezolid concentrations included higher weight, creatinine clearance rates, and fibrinogen and antithrombin concentrations, lower concentrations of lactate, and the presence of acute respiratory distress syndrome (ARDS). Linezolid clearance was increased in ARDS patients (by 82%) and in patients with elevated fibrinogen or decreased lactate concentrations. In simulated patients, most covariates, including fibrinogen and lactate concentrations and weight, showed quantitatively minor effects on target attainment (difference of ≤9% between the first and fourth quartiles of the respective parameters). In contrast, the presence of ARDS had the strongest influence, with only ≤6% of simulated patients reaching this target. In conclusion, the presence of ARDS was identified as a new and strong predictor of insufficient linezolid concentrations, which might cause treatment failure. Insufficient concentrations might also be a major problem in patients with combined alterations of other covariate parameters. (This study has been registered at ClinicalTrials.gov under registration number NCT01793012.).
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
Clinical Determinants of Target Non-Attainment of Linezolid in Plasma and Interstitial Space Fluid: A Pooled Population Pharmacokinetic Analysis with Focus on Critically Ill Patients.Clin Pharmacokinet. 2017 Jun;56(6):617-633. doi: 10.1007/s40262-016-0463-7. Clin Pharmacokinet. 2017. PMID: 27753002 Clinical Trial.
-
Role of renal function in risk assessment of target non-attainment after standard dosing of meropenem in critically ill patients: a prospective observational study.Crit Care. 2017 Oct 21;21(1):263. doi: 10.1186/s13054-017-1829-4. Crit Care. 2017. PMID: 29058601 Free PMC article. Clinical Trial.
-
Pharmacokinetic/pharmacodynamic evaluation of linezolid for the treatment of staphylococcal infections in critically ill patients.Int J Antimicrob Agents. 2016 Sep;48(3):259-64. doi: 10.1016/j.ijantimicag.2016.05.009. Epub 2016 Jul 5. Int J Antimicrob Agents. 2016. PMID: 27474469
-
Pharmacokinetics of linezolid in critically ill patients.Expert Opin Drug Metab Toxicol. 2016 Jun;12(6):595-600. doi: 10.1517/17425255.2016.1170807. Epub 2016 Apr 7. Expert Opin Drug Metab Toxicol. 2016. PMID: 27020789 Review.
-
A Review of Population Pharmacokinetic Analyses of Linezolid.Clin Pharmacokinet. 2022 Jun;61(6):789-817. doi: 10.1007/s40262-022-01125-2. Epub 2022 Jun 14. Clin Pharmacokinet. 2022. PMID: 35699914 Free PMC article. Review.
Cited by
-
Linezolid Concentrations in Plasma and Subcutaneous Tissue are Reduced in Obese Patients, Resulting in a Higher Risk of Underdosing in Critically Ill Patients: A Controlled Clinical Pharmacokinetic Study.J Clin Med. 2020 Apr 9;9(4):1067. doi: 10.3390/jcm9041067. J Clin Med. 2020. PMID: 32283731 Free PMC article.
-
Effect of Platelet Parameters on Linezolid-Related Thrombocytopenia in Hospitalized Patients.Infect Drug Resist. 2023 Sep 12;16:6145-6154. doi: 10.2147/IDR.S408102. eCollection 2023. Infect Drug Resist. 2023. PMID: 37719650 Free PMC article.
-
Dosage Strategy of Linezolid According to the Trough Concentration Target and Renal Function in Chinese Critically Ill Patients.Front Pharmacol. 2022 Apr 11;13:844567. doi: 10.3389/fphar.2022.844567. eCollection 2022. Front Pharmacol. 2022. PMID: 35479324 Free PMC article.
-
Pharmacokinetics of Linezolid Dose Adjustment for Creatinine Clearance in Critically Ill Patients: A Multicenter, Prospective, Open-Label, Observational Study.Drug Des Devel Ther. 2021 May 19;15:2129-2141. doi: 10.2147/DDDT.S303497. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 34040351 Free PMC article. Clinical Trial.
-
Dosage Individualization of Linezolid: Precision Dosing of Linezolid To Optimize Efficacy and Minimize Toxicity.Antimicrob Agents Chemother. 2021 May 18;65(6):e02490-20. doi: 10.1128/AAC.02490-20. Print 2021 May 18. Antimicrob Agents Chemother. 2021. PMID: 33820765 Free PMC article.
References
-
- Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. 2007. Occurrence and outcomes of sepsis: influence of race. Crit Care Med 35:763–768. doi:10.1097/01.CCM.0000256726.80998.BF. - DOI - PubMed
-
- Engel C, Brunkhorst FM, Bone HG, Brunkhorst R, Gerlach H, Grond S, Gruendling M, Huhle G, Jaschinski U, John S, Mayer K, Oppert M, Olthoff D, Quintel M, Ragaller M, Rossaint R, Stuber F, Weiler N, Welte T, Bogatsch H, Hartog C, Loeffler M, Reinhart K. 2007. Epidemiology of sepsis in Germany: results from a national prospective multicenter study. Intensive Care Med 33:606–618. doi:10.1007/s00134-006-0517-7. - DOI - PubMed
-
- Martin CM, Priestap F, Fisher H, Fowler RA, Heyland DK, Keenan SP, Longo CJ, Morrison T, Bentley D, Antman N, STAR Registry Investigators. 2009. A prospective, observational registry of patients with severe sepsis: the Canadian Sepsis Treatment and Response Registry. Crit Care Med 37:81–88. doi:10.1097/CCM.0b013e31819285f0. - DOI - PubMed
-
- Quenot JP, Binquet C, Kara F, Martinet O, Ganster F, Navellou JC, Castelain V, Barraud D, Cousson J, Louis G, Perez P, Kuteifan K, Noirot A, Badie J, Mezher C, Lessire H, Pavon A. 2013. The epidemiology of septic shock in French intensive care units: the prospective multicenter cohort EPISS study. Crit Care 17:R65. doi:10.1186/cc12598. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

