Adaptive immune response to lipoproteins of Staphylococcus aureus in healthy subjects
- PMID: 27324828
- PMCID: PMC5096053
- DOI: 10.1002/pmic.201600151
Adaptive immune response to lipoproteins of Staphylococcus aureus in healthy subjects
Abstract
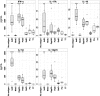
Staphylococcus aureus is a frequent commensal but also a dangerous pathogen, causing many forms of infection ranging from mild to life-threatening conditions. Among its virulence factors are lipoproteins, which are anchored in the bacterial cell membrane. Lipoproteins perform various functions in colonization, immune evasion, and immunomodulation. These proteins are potent activators of innate immune receptors termed Toll-like receptors 2 and 6. This study addressed the specific B-cell and T-cell responses directed to lipoproteins in human S. aureus carriers and non-carriers. 2D immune proteomics and ELISA approaches revealed that titers of antibodies (IgG) binding to S. aureus lipoproteins were very low. Proliferation assays and cytokine profiling data showed only subtle responses of T cells; some lipoproteins did not elicit proliferation. Hence, the robust activation of the innate immune system by S. aureus lipoproteins does not translate into a strong adaptive immune response. Reasons for this may include inaccessibility of lipoproteins for B cells as well as ineffective processing and presentation of the antigens to T cells.
Keywords: Antibody; Human; Lipoprotein; Microbiology; S. aureus; T cell.
© 2016 The Authors. Proteomics Published by Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



Comment in
-
Lipoprotein immunoproteomics question the potential of Staphylococcus aureus TLR2 agonists as vaccine antigens.Proteomics. 2016 Oct;16(20):2603-2604. doi: 10.1002/pmic.201600351. Proteomics. 2016. PMID: 27667303
-
Does the choice of forceps during IUD insertion affect pain scores?Practitioner. 2016 Nov;260(1798):9. Practitioner. 2016. PMID: 28968047 No abstract available.
References
-
- Wertheim, H. F. , Melles, D. C. , Vos, M. C. , van Leeuwen, W. et al., The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 2005, 5, 751–762. - PubMed
-
- Bröker, B. M. , Holtfreter, S. , Bekeredjian‐Ding, I. , Immune control of Staphylococcus aureus—regulation and counter‐regulation of the adaptive immune response. Int. J. Med. Microbiol. 2014, 304, 204–214. - PubMed
-
- Salgado‐Pabon, W. , Schlievert, P. M. , Models matter: the search for an effective Staphylococcus aureus vaccine. Nat. Rev. Microbiol. 2014, 12, 585–591. - PubMed
-
- van Belkum, A. , Verkaik, N. J. , de Vogel, C. P. , Boelens, H. A. et al., Reclassification of Staphylococcus aureus nasal carriage types. J. Infect. Dis. 2009, 199, 1820–1826. - PubMed
-
- Boyle‐Vavra, S. , Daum, R. S. , Community‐acquired methicillin‐resistant Staphylococcus aureus: the role of Panton‐Valentine leukocidin. Lab. Invest. 2007, 87, 3–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

