Cervical dystonia: a neural integrator disorder
- PMID: 27324878
- PMCID: PMC5840887
- DOI: 10.1093/brain/aww141
Cervical dystonia: a neural integrator disorder
Abstract
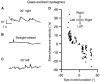
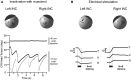
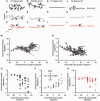
Ocular motor neural integrators ensure that eyes are held steady in straight-ahead and eccentric positions of gaze. Abnormal function of the ocular motor neural integrator leads to centripetal drifts of the eyes with consequent gaze-evoked nystagmus. In 2002 a neural integrator, analogous to that in the ocular motor system, was proposed for the control of head movements. Recently, a counterpart of gaze-evoked eye nystagmus was identified for head movements; in which the head could not be held steady in eccentric positions on the trunk. These findings lead to a novel pathophysiological explanation in cervical dystonia, which proposed that the abnormalities of head movements stem from a malfunctioning head neural integrator, either intrinsically or as a result of impaired cerebellar, basal ganglia, or peripheral feedback. Here we briefly recapitulate the history of the neural integrator for eye movements, then further develop the idea of a neural integrator for head movements, and finally discuss its putative role in cervical dystonia. We hypothesize that changing the activity in an impaired head neural integrator, by modulating feedback, could treat dystonia.
Keywords: cerebellum; integrator; midbrain; nystagmus; tremor.
© The Author (2016). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures

Comment in
-
Reply: Contributions of visual and motor signals in cervical dystonia.Brain. 2017 Jan;140(1):e5. doi: 10.1093/brain/aww292. Epub 2016 Dec 19. Brain. 2017. PMID: 27993889 No abstract available.
-
Contributions of visual and motor signals in cervical dystonia.Brain. 2017 Jan;140(1):e4. doi: 10.1093/brain/aww282. Epub 2016 Dec 19. Brain. 2017. PMID: 27993890 No abstract available.
References
-
- Aksay E, Gamkrelidze G, Seung HS, Baker R, Tank DW . In vivo intracellular recording and perturbation of persistent activity in a neural integrator . Nat Neurosci 2001. ; 4 : 184 – 93 . - PubMed
-
- Andersen RA, Snyder LH, Li CS, Stricanne B . Coordinate transformations in the representation of spatial information . Curr Opin Neurobiol 1993. ; 3 : 171 – 6 . - PubMed
-
- Arnold DB, Robinson DA . A learning network model of the neural integrator of the oculomotor system . Biol Cybern 1991. ; 64 : 447 – 54 . - PubMed
-
- Arnold DB, Robinson DA, Leigh RJ . Nystagmus induced by pharmacological inactivation of the brainstem ocular motor integrator in monkey . Vision Res 1999. ; 39 : 4286 – 95 . - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

