Synthesis and stereochemical determination of an antiparasitic pseudo-aminal type monoterpene indole alkaloid
- PMID: 27324906
- PMCID: PMC4935745
- DOI: 10.1007/s11418-016-1012-2
Synthesis and stereochemical determination of an antiparasitic pseudo-aminal type monoterpene indole alkaloid
Abstract
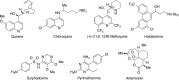
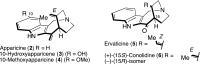
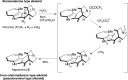
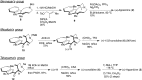
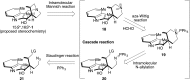
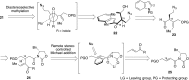
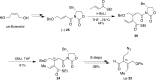
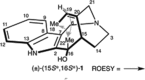
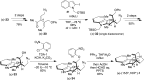
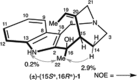
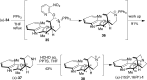
5-Nor stemmadenine alkaloids, isolated from the genus Tabernaemontana, display a range of bioactivity. 16-Hydroxy-16,22-dihydroapparicine, the active component of an extract from the Tabernaemontana sp. (dichotoma, elegans, and divaricate), exhibited potent antimalarial activity, representing the first such report of the antimalarial property of 5-nor stemmadenine alkaloids. We, therefore, decided to attempt the total synthesis of the compound to explore its antimalarial activity and investigate structure and bioactivity relationships. As a result, we completed the first total synthesis of 16-hydroxy-16,22-dihydroapparicine, by combining a phosphine-mediated cascade reaction, diastereoselective nucleophilic addition of 2-acylindole or methylketone via a Felkin-Anh transition state, and chirality transferring intramolecular Michael addition. We also clarified the absolute stereochemistries of the compound. Furthermore, we evaluated the activity of the synthetic compound, as well as that of some intermediates, all of which showed weak activity against chloroquine-resistant Plasmodium falciparum (K1 strain) malaria parasites.
Keywords: 5-Nor stemmadenine alkaloid; Antimalarial agent; Chirality transfer intramolecular Michael reaction; Diastereoselective 1,2-addition using indole nucleophile; Iminophosphorane mediated cascade reaction; Pseudo-aminal type structure.
Figures


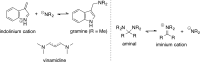




References
-
- Ōmura S. The search for bioactive compounds from microorganisms. Brock/Springer series in contemporary biosciences. New York: Springer-Verlag; 1992.
-
- Ōmura S. Splendid gifts from microorganisms. 5. Tokyo: The Kitasato Institute; 2015.
-
- World Health Organization (WHO) (2014) World malaria report 2014. Available online at: http://www.who.int/malaria/publications/world_malaria_report_2014/report.... Accessed 1 Apr 2016
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

