Reversible S-nitrosylation in an engineered azurin
- PMID: 27325093
- PMCID: PMC4918514
- DOI: 10.1038/nchem.2489
Reversible S-nitrosylation in an engineered azurin
Abstract
S-Nitrosothiols are known as reagents for NO storage and transportation and as regulators in many physiological processes. Although the S-nitrosylation catalysed by haem proteins is well known, no direct evidence of S-nitrosylation in copper proteins has been reported. Here, we report reversible insertion of NO into a copper-thiolate bond in an engineered copper centre in Pseudomonas aeruginosa azurin by rational design of the primary coordination sphere and tuning its reduction potential by deleting a hydrogen bond in the secondary coordination sphere. The results not only provide the first direct evidence of S-nitrosylation of Cu(II)-bound cysteine in metalloproteins, but also shed light on the reaction mechanism and structural features responsible for stabilizing the elusive Cu(I)-S(Cys)NO species. The fast, efficient and reversible S-nitrosylation reaction is used to demonstrate its ability to prevent NO inhibition of cytochrome bo3 oxidase activity by competing for NO binding with the native enzyme under physiologically relevant conditions.
Figures
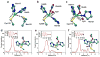


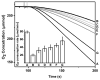
Comment in
-
Now we NO.Nat Chem. 2016 Jun 21;8(7):635. doi: 10.1038/nchem.2562. Nat Chem. 2016. PMID: 27325084 No abstract available.
Similar articles
-
Azurin: A Model to Study a Metal Coordination Sphere or Electron Transfer in Metalloproteins.Int J Mol Sci. 2025 Apr 26;26(9):4125. doi: 10.3390/ijms26094125. Int J Mol Sci. 2025. PMID: 40362365 Free PMC article. Review.
-
Engineering a Hetero-Bimetallic Azurin Photoenzyme for Photoredox Nitrite Reduction and SNO Adduct Formation.Chemistry. 2025 Apr 15;31(22):e202500143. doi: 10.1002/chem.202500143. Epub 2025 Mar 15. Chemistry. 2025. PMID: 40038049
-
Primary and Secondary Coordination Sphere Effects on the Structure and Function of S-Nitrosylating Azurin.J Am Chem Soc. 2023 Sep 20;145(37):20610-20623. doi: 10.1021/jacs.3c07399. Epub 2023 Sep 11. J Am Chem Soc. 2023. PMID: 37696009 Free PMC article.
-
Spin-density distribution in the copper site of azurin.Chemphyschem. 2006 Jun 12;7(6):1286-93. doi: 10.1002/cphc.200500551. Chemphyschem. 2006. PMID: 16683281
-
The role of ligand-containing loops at copper sites in proteins.Nat Prod Rep. 2008 Feb;25(1):15-24. doi: 10.1039/b707987g. Epub 2007 Oct 8. Nat Prod Rep. 2008. PMID: 18250895 Review.
Cited by
-
The Periodic Table's Impact on Bioinorganic Chemistry and Biology's Selective Use of Metal Ions.Struct Bond. 2019;182:153-173. doi: 10.1007/430_2019_45. Epub 2019 Oct 5. Struct Bond. 2019. PMID: 36567794 Free PMC article.
-
Clarifying the Copper Coordination Environment in a de Novo Designed Red Copper Protein.Inorg Chem. 2018 Oct 1;57(19):12291-12302. doi: 10.1021/acs.inorgchem.8b01989. Epub 2018 Sep 18. Inorg Chem. 2018. PMID: 30226758 Free PMC article.
-
Stepwise nitrosylation of the nonheme iron site in an engineered azurin and a molecular basis for nitric oxide signaling mediated by nonheme iron proteins.Chem Sci. 2021 Mar 31;12(19):6569-6579. doi: 10.1039/d1sc00364j. Chem Sci. 2021. PMID: 34040732 Free PMC article.
-
Catalysis and Electron Transfer in De Novo Designed Metalloproteins.Chem Rev. 2022 Jul 27;122(14):12046-12109. doi: 10.1021/acs.chemrev.1c01025. Epub 2022 Jun 28. Chem Rev. 2022. PMID: 35763791 Free PMC article. Review.
-
Contrasting secondary coordination sphere effects on spin density distribution in Red vs. Blue Cu azurin.J Biol Inorg Chem. 2025 Aug;30(4-5):397-410. doi: 10.1007/s00775-025-02116-x. Epub 2025 May 24. J Biol Inorg Chem. 2025. PMID: 40413301
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

