Real-World Data Collection Regarding Titration Algorithms for Insulin Glargine in Patients With Type 2 Diabetes Mellitus
- PMID: 27325389
- PMCID: PMC5032964
- DOI: 10.1177/1932296816654714
Real-World Data Collection Regarding Titration Algorithms for Insulin Glargine in Patients With Type 2 Diabetes Mellitus
Abstract
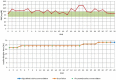
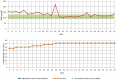
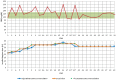
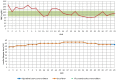
The primary objective of this study was to collect data regarding the effectiveness of different dose titration algorithms (TAs) for optimization or initiation of basal insulin supported oral therapy (BOT) in patients with type 2 diabetes. A total of 50 patients were enrolled in this trial (17 women, 33 men, age 63 ± 8 years, HbA1c 7.9 ± 0.8%). The investigator decided on an individual basis to apply any of 4 standard TAs: standard (S: fasting glucose target 90-130 mg/dL, n = 39), standard-fast titration (S-FT: 90-130 mg/dL, larger dose increments at FBG < 180 mg/dl, n = 1), less tight (LT: 110-150 mg/dL, n = 5), and tight (T: 70-100 mg/dL, n = 5). During the next 30 days daily contacts were used to adapt the insulin dose. The majority of all patients (70%) achieved a stable insulin glargine dose within 5 ± 6 days after initiation of the dose titration. HbA1c improved from 7.9 ± 0.8% to 7.5 ± 0.7% (P < .001). In total, 1300 dose decisions were made (1192 according to the TA and 108 by the physicians independently from the TA in 29 patients [58% of study population]). Reasons for TA-overruling dosing decisions were hypoglycemic events (14 mild/4 moderate) in 9 patients. In the majority of these cases (89.8%), the physician recommended continuation of the previous dose or a higher dose. The majority of FBG values were within the respective target range after 4 weeks. In conclusion, the insulin glargine TAs delivered safe dose recommendations with a low risk of hypoglycemia, which successfully led to a stable dose in the vast majority of patients.
Keywords: basal insulin; fasting blood glucose; titration algorithm.
© 2016 Diabetes Technology Society.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AP, BS, KF, HP, LR, and DT have received speaker and consultancy fees, research grants, and travel support from Sanofi. JS and FF are employees of Sanofi.
Figures
References
-
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2012;55:1577-1596. - PubMed
-
- Levien TL, Baker DE, White JR, Jr, Campbell RK. Insulin glargine: a new basal insulin. Ann Pharmacother. 2002; 36:1019-1027. - PubMed
-
- Gough SC, Harris S, Woo V, Davies M. Insulin degludec: overview of a novel ultra long-acting basal insulin. Diabetes Obes Metab. 2013;15:301-309. - PubMed
-
- Goldman-Levine JD, Lee KW. Insulin detemir—a new basal insulin analog. Ann Pharmacother. 2005;39:502-507. - PubMed
-
- Sutton G, Minguet J, Ferrero C, Bramlage P. U300, a novel long-acting insulin formulation. Expert Opin Biol Ther. 2014;14:1849-1860. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

