GATA6 regulates EMT and tumour dissemination, and is a marker of response to adjuvant chemotherapy in pancreatic cancer
- PMID: 27325420
- PMCID: PMC5070637
- DOI: 10.1136/gutjnl-2015-311256
GATA6 regulates EMT and tumour dissemination, and is a marker of response to adjuvant chemotherapy in pancreatic cancer
Abstract
Background and aims: The role of GATA factors in cancer has gained increasing attention recently, but the function of GATA6 in pancreatic ductal adenocarcinoma (PDAC) is controversial. GATA6 is amplified in a subset of tumours and was proposed to be oncogenic, but high GATA6 levels are found in well-differentiated tumours and are associated with better patient outcome. By contrast, a tumour-suppressive function of GATA6 was demonstrated using genetic mouse models. We aimed at clarifying GATA6 function in PDAC.
Design: We combined GATA6 silencing and overexpression in PDAC cell lines with GATA6 ChIP-Seq and RNA-Seq data, in order to understand the mechanism of GATA6 functions. We then confirmed some of our observations in primary patient samples, some of which were included in the ESPAC-3 randomised clinical trial for adjuvant therapy.
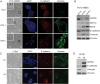
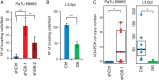
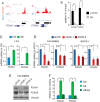
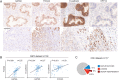
Results: GATA6 inhibits the epithelial-mesenchymal transition (EMT) in vitro and cell dissemination in vivo. GATA6 has a unique proepithelial and antimesenchymal function, and its transcriptional regulation is direct and implies, indirectly, the regulation of other transcription factors involved in EMT. GATA6 is lost in tumours, in association with altered differentiation and the acquisition of a basal-like molecular phenotype, consistent with an epithelial-to-epithelial (ET2) transition. Patients with basal-like GATA6low tumours have a shorter survival and have a distinctly poor response to adjuvant 5-fluorouracil (5-FU)/leucovorin. However, modulation of GATA6 expression in cultured cells does not directly regulate response to 5-FU.
Conclusions: We provide mechanistic insight into GATA6 tumour-suppressive function, its role as a regulator of canonical epithelial differentiation, and propose that loss of GATA6 expression is both prognostic and predictive of response to adjuvant therapy.
Keywords: ADJUVANT TREATMENT; CANCER GENETICS; EPITHELIAL DIFFERENTIATION; GENE REGULATION; PANCREATIC CANCER.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- MC_PC_15080/MRC_/Medical Research Council/United Kingdom
- 16186/CRUK_/Cancer Research UK/United Kingdom
- SGP/1/CSO_/Chief Scientist Office/United Kingdom
- 8968/CRUK_/Cancer Research UK/United Kingdom
- 15957/CRUK_/Cancer Research UK/United Kingdom
- PB-PG-0407-13363/DH_/Department of Health/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- 11883/CRUK_/Cancer Research UK/United Kingdom
- MR/N005813/1/MRC_/Medical Research Council/United Kingdom
- 17680/CRUK_/Cancer Research UK/United Kingdom
- 17263/CRUK_/Cancer Research UK/United Kingdom
- 16812/CRUK_/Cancer Research UK/United Kingdom
- P 27361/FWF_/Austrian Science Fund FWF/Austria
- 08/29/02/DH_/Department of Health/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
