Follicular helper T cells mediate IgE antibody response to airborne allergens
- PMID: 27325434
- PMCID: PMC5115999
- DOI: 10.1016/j.jaci.2016.04.021
Follicular helper T cells mediate IgE antibody response to airborne allergens
Abstract
Background: TH2 cells have long been believed to play a pivotal role in allergic immune responses, including IgE antibody production and type 2 cytokine-mediated inflammation and pathology. A new T-cell subset, follicular helper T (TFH) cells, is specialized in supporting B-cell maturation and antibody production.
Objective: We sought to investigate the roles of TFH cells in allergic immune responses.
Methods: Naive mice were exposed to cytokines or natural allergens through the airways. Development of allergic immune responses was analyzed by collecting draining lymph nodes and sera and by challenging the animals. Cytokine reporter mice and gene-deficient mice were used to dissect the immunologic mechanisms.
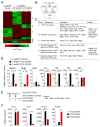
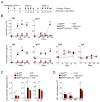
Results: We observed the development of IL-4-producing TFH cells and TH2 cells in draining lymph nodes after airway exposure to IL-1 family cytokines or natural allergens. TFH and TH2 cells demonstrated unique phenotypes, tissue localization, and cytokine responses. TFH cells supported the sustained production of IgE antibody in vivo in the absence of other T-cell subsets or even when TH2 cell functions were severely compromised. Conversely, conditional deficiency of the master regulator Bcl6 in CD4+ T cells resulted in a marked reduction in TFH cell numbers and IgE antibody levels, but type 2 cytokine responses and eosinophilic inflammation in the airways remained unaffected.
Conclusion: TFH cells play critical roles in the regulation of IgE antibody production. Allergic immune responses to airborne allergens likely involve 2 distinct subsets of IL-4-producing CD4+ T cells, namely TFH and Th2 cells.
Keywords: Follicular T cells; IL-4; IgE; T(H)2 cells; allergens; allergy.
Copyright © 2016 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. - PubMed
-
- McKenzie AN, Spits H, Eberl G. Innate lymphoid cells in inflammation and immunity. Immunity. 2014;41:366–374. - PubMed
-
- Corry DB, Kheradmand F. Induction and regulation of the IgE response. Nature. 1999;402:B18–23. - PubMed
-
- Finkelman FD, Holmes J, Katona IM, Urban JF, Jr, Beckmann MP, Park LS, et al. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. - PubMed
-
- Bousquet J, Anto JM, Bachert C, Bousquet PJ, Colombo P, Crameri R, et al. Factors responsible for differences between asymptomatic subjects and patients presenting an IgE sensitization to allergens. A GA2LEN project. Allergy. 2006;61:671–680. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

