Low-Dose Oral Cyclophosphamide and Methotrexate Maintenance for Hormone Receptor-Negative Early Breast Cancer: International Breast Cancer Study Group Trial 22-00
- PMID: 27325862
- PMCID: PMC5035120
- DOI: 10.1200/JCO.2015.65.6595
Low-Dose Oral Cyclophosphamide and Methotrexate Maintenance for Hormone Receptor-Negative Early Breast Cancer: International Breast Cancer Study Group Trial 22-00
Abstract
Purpose: To evaluate the benefit of low-dose cyclophosphamide and methotrexate (CM) maintenance, which previously demonstrated antitumor activity and few adverse effects in advanced breast cancer, in early breast cancer.
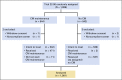
Patients and methods: International Breast Cancer Study Group (IBCSG) Trial 22-00, a randomized phase III clinical trial, enrolled 1,086 women (1,081 intent-to-treat) from November 2000 to December 2012. Women with estrogen receptor- and progesterone receptor-negative (< 10% positive cells by immunohistochemistry) early breast cancer any nodal and human epidermal growth factor receptor 2 status, were randomly assigned anytime between primary surgery and 56 days after the first day of last course of adjuvant chemotherapy to CM maintenance (cyclophosphamide 50 mg/day orally continuously and methotrexate 2.5 mg twice/day orally on days 1 and 2 of every week for 1 year) or to no CM. The primary end point was disease-free survival (DFS), which included invasive recurrences, second (breast and nonbreast) malignancies, and deaths.
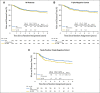
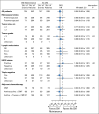
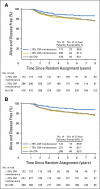
Results: After a median of 6.9 years of follow-up, DFS was not significantly better for patients assigned to CM maintenance compared with patients assigned to no CM, both overall (hazard ratio [HR], 0.84; 95% CI, 0.66 to 1.06;P = .14) and in triple-negative (TN) disease (n = 814; HR, 0.80; 95% CI, 0.60 to 1.06). Patients with TN, node-positive disease had a nonstatistically significant reduced HR (n = 340; HR, 0.72; 95% CI, 0.49 to 1.05). Seventy-one (13%) of 542 patients assigned to CM maintenance did not start CM. Of 473 patients who received at least one CM maintenance dose (including two patients assigned to no CM), 64 (14%) experienced a grade 3 or 4 treatment-related adverse event; elevated serum transaminases was the most frequently reported (7%), followed by leukopenia (2%).
Conclusion: CM maintenance did not produce a significant reduction in DFS events in hormone receptor-negative early breast cancer. The trend toward benefit observed in the TN, node-positive subgroup supports additional exploration of this strategy in the TN, higher-risk population.
Trial registration: ClinicalTrials.gov NCT00022516.
© 2016 by American Society of Clinical Oncology.
Conflict of interest statement
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Figures
Comment in
-
Chemotherapy for Triple-Negative Breast Cancer: Is More Better?J Clin Oncol. 2016 Oct 1;34(28):3369-71. doi: 10.1200/JCO.2016.68.4068. Epub 2016 Aug 22. J Clin Oncol. 2016. PMID: 27551109 No abstract available.
-
Reply to L. Moscetti.J Clin Oncol. 2017 May 10;35(14):1628. doi: 10.1200/JCO.2016.71.5730. Epub 2017 Jan 17. J Clin Oncol. 2017. PMID: 28095153 No abstract available.
-
Are All Patients Truly Hormone Receptor Negative?J Clin Oncol. 2017 May 10;35(14):1627. doi: 10.1200/JCO.2016.69.2343. Epub 2017 Jan 17. J Clin Oncol. 2017. PMID: 28095158 No abstract available.
References
-
- Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14:2738–2746. - PubMed
-
- Colleoni M, Cole BF, Viale G, et al. Classical cyclophosphamide, methotrexate, and fluorouracil chemotherapy is more effective in triple-negative, node-negative breast cancer: Results from two randomized trials of adjuvant chemoendocrine therapy for node-negative breast cancer. J Clin Oncol. 2010;28:2966–2973. - PMC - PubMed
-
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

