Vaginal progesterone on the prevention of preterm birth and neonatal complications in high risk women: A randomized placebo-controlled double-blind study
- PMID: 27326415
- PMCID: PMC4910039
Vaginal progesterone on the prevention of preterm birth and neonatal complications in high risk women: A randomized placebo-controlled double-blind study
Abstract
Background: Preterm birth is the major cause of neonatal mortality and morbidity.
Objective: The aim of this study was to evaluate the effect of prophylactic vaginal progesterone on decreasing preterm birth rate and neonatal complications in a high-risk population.
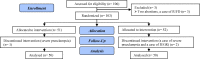
Materials and methods: A randomized, double-blind, placebo-controlled study was performed on 100 high-risk singleton pregnancies. Vaginal suppository progesterone (400 mg) or placebo was administered daily between 16-22 wks to 36 wks of gestation. Progesterone (n=50) and placebo (n=50) groups were compared for incidence of preterm delivery and neonatal complications.
Results: The preterm birth rate was 52%. Preterm birth rate before the 37 wks of gestation (68% vs. 36%: RR=1.89, 95% CI: 1.25-2.86) and also before the 34 wks of gestation (42% vs. 18%: RR=2.33, 95% CI: 1.19-4.58) in placebo group was significantly higher than progesterone group. Our study also showed that the administration of vaginal progesterone was associated with a significant reduction in the risk of birth weight ≤2500 gr, the rates of respiratory distress syndrome (RDS) and admission to the Neonatal Intensive Care Unit (NICU) in the progesterone group when compared with the placebo group. However, there was no significant difference between the two groups in terms of neonatal death, days of admission in NICU, intraventricular hemorrhage and necrotizing enterocolitis.
Conclusion: Prophylactic vaginal progesterone reduced the rate of preterm delivery, the risk of a birth weight ≤2500 gr, the rates of RDS and admission to NICU in women who were at risk of preterm delivery.
Keywords: Premature birth; Prevention; Progesterone; Randomized controlled trial.
Conflict of interest statement
There is no conflict of interest in this study.
References
-
- Cunningham FG, Leveno KJ, Bloom SL, Hauth JC, Rouse DJ, Spong CY, editors . Williams Obstetrics. 24th Ed. New York: McGraw-Hill Medical; 2014.
-
- Mathews J, McDonald MF. Infant mortality statistics from the 2008 period linked birth infant death data sct. Natl Vital Stat Rep. 2012;80:1–49. - PubMed
-
- Hamilton BE, Martin JA, Ventura SJ. Births: preliminary data for 2010. Natl Vital Stat Rep. 2011;60:1–26. - PubMed
-
- Martison DR, Damus K, Fiore E, Petrini J, Alter C. Preterm delivery: a public health perspective. Paediatr Perinat Epidemiol . 2001;15 (Suppl.):7–16. - PubMed
-
- Gluckman PD, Hanson MA. Living with the past: evolution, development and patterns of disease. Science. 2004;305:1733–1736. - PubMed
LinkOut - more resources
Full Text Sources
Medical