The biological response to orthopaedic implants for joint replacement: Part I: Metals
- PMID: 27328111
- PMCID: PMC5537059
- DOI: 10.1002/jbm.b.33734
The biological response to orthopaedic implants for joint replacement: Part I: Metals
Abstract
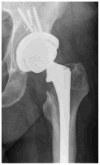
Joint replacement is a commonly performed, highly successful orthopaedic procedure, for which surgeons have a large choice of different materials and implant designs. The materials used for joint replacement must be both biologically acceptable to minimize adverse local tissue reactions, and robust enough to support weight bearing during common activities of daily living. Modern joint replacements are made from metals and their alloys, polymers, ceramics, and composites. This review focuses on the biological response to the different biomaterials used for joint replacement. In general, modern materials for joint replacement are well tolerated by the body as long as they are in bulk (rather than in particulate or ionic) form, are mechanically stable and noninfected. If the latter conditions are not met, the prosthesis will be associated with an acute/chronic inflammatory reaction, peri-prosthetic osteolysis, loosening and failure. This article (Part 1 of 2) is dedicated to the use of metallic devices in orthopaedic surgery including the associated biological response to metallic byproducts is a review of the basic science literature regarding this topic. © 2016 Wiley Periodicals, Inc. J Biomed Mater Res Part B: Appl Biomater, 105B: 2162-2173, 2017.
Keywords: biological response; biomaterials; foreign body response; inflammation; orthopaedic implants.
© 2016 Wiley Periodicals, Inc.
Figures

References
-
- Richards RG, Moriarty TF, Miclau T, McClellan RT, Grainger DW. Advances in biomaterials and surface technologies. J Orthop Trauma. 2012;26:703–707. - PubMed
-
- Epinette JA, Manley MT. Hydroxyapatite-coated total knee replacement: Clinical experience at 10 to 15 years. J Bone Joint Surg Br. 2007;89:34–38. - PubMed
-
- Attar FG, Khaw F-M, Kirk LMG, Gregg PJ. Survivorship analysis at 15 years of cemented press-fit condylar total knee arthroplasty. J Arthroplasty. 2008;23:344–349. - PubMed
-
- Melton JTK, Mayahi R, Baxter SE, Facek M, Glezos C. Long-term outcome in an uncemented, hydroxyapatite-coated total knee replacement: A 15- to 18-year survivorship analysis. J Bone Joint Surg Br. 2012;94:1067–1070. - PubMed
-
- El Masri F, Kerboull L, Kerboull M, Courpied JP, Hamadouche M. Is the so-called ‘French paradox’ a reality? Long-term survival and migration of the Charnley–Kerboull stem cemented line-to-line. J Bone Joint Surg Br. 2010;92:342–348. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

