The Discovery and Early Days of TGF-β: A Historical Perspective
- PMID: 27328871
- PMCID: PMC4930926
- DOI: 10.1101/cshperspect.a021865
The Discovery and Early Days of TGF-β: A Historical Perspective
Abstract
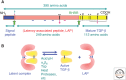
Transforming growth factors (TGFs) were discovered as activities that were secreted by cancer cells, and later by normal cells, and had the ability to phenotypically and reversibly transform immortalized fibroblasts. TGF-β distinguished itself from TGF-α because it did not bind to the same epidermal growth factor (EGF) receptor as TGF-α and, therefore, acted through different cell-surface receptors and signaling mediators. This review summarizes the discovery of TGF-β, the early developments in its molecular and biological characterization with its many biological activities in different cell and tissue contexts and its roles in disease, the realization that there is a family of secreted TGF-β-related proteins with many differentiation functions in development and activities in normal cell and tissue physiology, and the subsequent identification and characterization of the receptors and effectors that mediate TGF-β family signaling responses.
Copyright © 2016 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures




References
-
- Akhurst RJ, Derynck R. 2001. TGF-β signaling in cancer—A double-edged sword. Trends Cell Biol 11: S44–S51. - PubMed
-
- Anzano MA, Roberts AB, Smith JM, Lamb LC, Sporn MB. 1982. Purification by reverse-phase high-performance liquid chromatography of an epidermal growth factor-dependent transforming growth factor. Anal Biochem 125: 217–224. - PubMed
-
- Assoian RK, Komoriya A, Meyers CA, Miller DM, Sporn MB. 1983. Transforming growth factor β in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem 258: 7155–7160. - PubMed
-
- Attisano L, Wrana JL, Cheifetz S, Massagué J. 1992. Novel activin receptors: Distinct genes and alternative mRNA splicing generate a repertoire of serine/threonine kinase receptors. Cell 68: 97–108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
