Estradiol does not affect spasms in the betamethasone-NMDA rat model of infantile spasms
- PMID: 27328917
- PMCID: PMC10765244
- DOI: 10.1111/epi.13434
Estradiol does not affect spasms in the betamethasone-NMDA rat model of infantile spasms
Abstract
Objective: This study attempted to validate the effects of neonatal estradiol in ameliorating the spasms in the prenatally betamethasone-primed N-methyl-d-aspartate (NMDA) model of infantile spasms in rats as shown previously in a mouse Arx gene knock-in expansion model of infantile spasms.
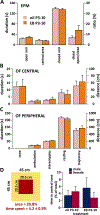
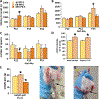
Methods: Neonatal rats prenatally exposed to betamethasone (on day 15 of pregnancy) were treated with subcutaneous 40 ng/g estradiol benzoate (EB) between postnatal days (P)3-P10 or P0-P5. A synthetic estrogen analogue, diethylstilbestrol, was used between P0 and P5 (2 μg per rat, s.c.). On P12, P13, and P15, the rats were subjected to NMDA-triggered spasms, and latency to onset and number of spasms were evaluated. Rats with EB on P3-P10 were tested after spasms in the open field, novel object recognition, and elevated plus maze to determine effects of treatment on behavior. Additional rats with P3-P10 or P0-P5 EB were investigated for γ-aminobutyric acid (GABA)ergic neurons (glutamate decarboxylase [GAD]67 expression) in the neocortex. As a positive control, a group of rats received either subcutaneous adrenocorticotropic hormone (ACTH) (2 × 0.3 mg/kg on P12 and 3 × 0.3 mg/kg on P13 and P14) or vehicle after the first episode of spasms on P12.
Results: Neither EB treatment nor diethylstilbestrol consistently affected expression of spasms in this model, although we found a significant increase in GAD67-immunopositive cells in the neocortex after P3-P10 and P0-P5 EB treatment, consistent with a study in mice. Behavioral tests showed increase in lateralization in male rats treated with P3-P10 EB, a behavioral trait usually associated with female sex. Diethylstilbestrol treatment in male rats resulted in arrested pubertal descent of testes. ACTH had robust effects in suppressing spasms.
Significance: Treatment of infantile spasms (IS) using neonatal EB may be justified in those cases of IS that present with detectable deficits in GABAergic neurons. In other types of IS, the efficacy of neonatal EB and its analogues is not supported.
Keywords: ACTH; Behavior; Diethylstilbestrol; Epileptic spasms; Validation.
Wiley Periodicals, Inc. © 2016 International League Against Epilepsy.
Conflict of interest statement
DISCLOSURE OF CONFLICT OF INTEREST
All other authors have no conflict of interest.
Figures



References
-
- Dulac O, Soufflet C, Chiron C, et al. What is West syndrome? Int Rev Neurobiol. 2002;49:1–22. - PubMed
-
- Riikonen R. Epidemiological data of West syndrome in Finland. Brain Dev. 2001;23:539–41. - PubMed
-
- Widjaja E, Go C, McCoy B, et al. Neurodevelopmental outcome of infantile spasms: A systematic review and meta-analysis. Epilepsy Res. 2015;109:155–62. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

