Immunotherapy for human papillomavirus-associated disease and cervical cancer: review of clinical and translational research
- PMID: 27329199
- PMCID: PMC4944018
- DOI: 10.3802/jgo.2016.27.e51
Immunotherapy for human papillomavirus-associated disease and cervical cancer: review of clinical and translational research
Abstract
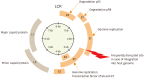
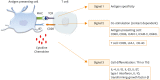
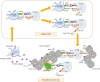
Cervical cancer is the fourth most lethal women's cancer worldwide. Current treatments against cervical cancer include surgery, radiotherapy, chemotherapy, and anti-angiogenic agents. However, despite the various treatments utilized for the treatment of cervical cancer, its disease burden remains a global issue. Persistent infection of human papillomavirus (HPV) has been identified as an essential step of pathogenesis of cervical cancer and many other cancers, and nation-wide HPV screening as well as preventative HPV vaccination program have been introduced globally. However, even though the commercially available prophylactic HPV vaccines, Gardasil (Merck) and Cervarix (GlaxoSmithKline), are effective in blocking the entry of HPV into the epithelium of cervix through generation of HPV-specific neutralizing antibodies, they cannot eliminate the pre-existing HPV infection. For these reason, other immunotherapeutic options against HPV-associated diseases, including therapeutic vaccines, have been continuously explored. Therapeutic HPV vaccines enhance cell-mediated immunity targeting HPV E6 and E7 antigens by modulating primarily dendritic cells and cytotoxic T lymphocyte. Our review will cover various therapeutic vaccines in development for the treatment of HPV-associated lesions and cancers. Furthermore, we will discuss the potential of immune checkpoint inhibitors that have recently been adopted and tested for their treatment efficacy against HPV-induced cervical cancer.
Keywords: Human Papillomavirus; Immune Checkpoint Inhibitor; Immunotherapy; Therapeutics; Uterine Cervical Neoplasms; Vaccines.
Conflict of interest statement
Figures
References
-
- Wakeham K, Kavanagh K. The burden of HPV-associated anogenital cancers. Curr Oncol Rep. 2014;16:402. - PubMed
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. - PubMed
-
- Cibula D, Abu-Rustum NR, Benedetti-Panici P, Köhler C, Raspagliesi F, Querleu D, et al. New classification system of radical hysterectomy: emphasis on a three-dimensional anatomic template for parametrial resection. Gynecol Oncol. 2011;122:264–268. - PubMed
-
- Rose PG. Concurrent chemoradiation for locally advanced carcinoma of the cervix: where are we in 2006? Ann Oncol. 2006;17(Suppl 10):x224–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

