Lycopene overproduction in Saccharomyces cerevisiae through combining pathway engineering with host engineering
- PMID: 27329233
- PMCID: PMC4915043
- DOI: 10.1186/s12934-016-0509-4
Lycopene overproduction in Saccharomyces cerevisiae through combining pathway engineering with host engineering
Abstract
Background: Microbial production of lycopene, a commercially and medically important compound, has received increasing concern in recent years. Saccharomyces cerevisiae is regarded as a safer host for lycopene production than Escherichia coli. However, to date, the lycopene yield (mg/g DCW) in S. cerevisiae was lower than that in E. coli and did not facilitate downstream extraction process, which might be attributed to the incompatibility between host cell and heterologous pathway. Therefore, to achieve lycopene overproduction in S. cerevisiae, both host cell and heterologous pathway should be delicately engineered.
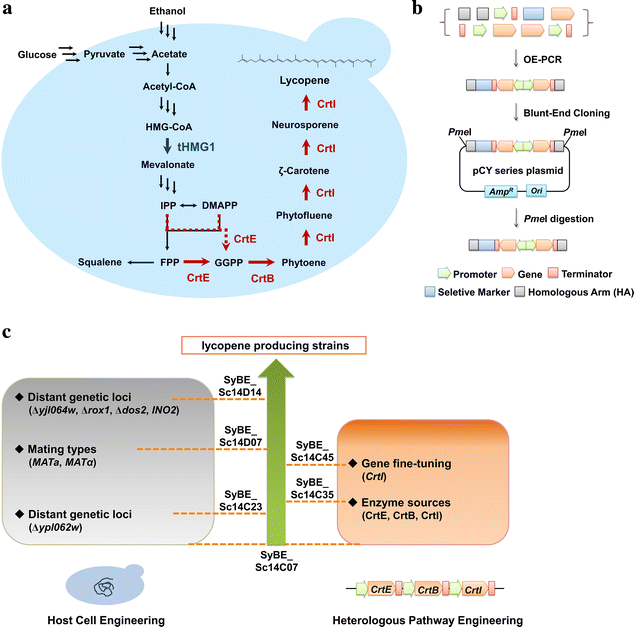
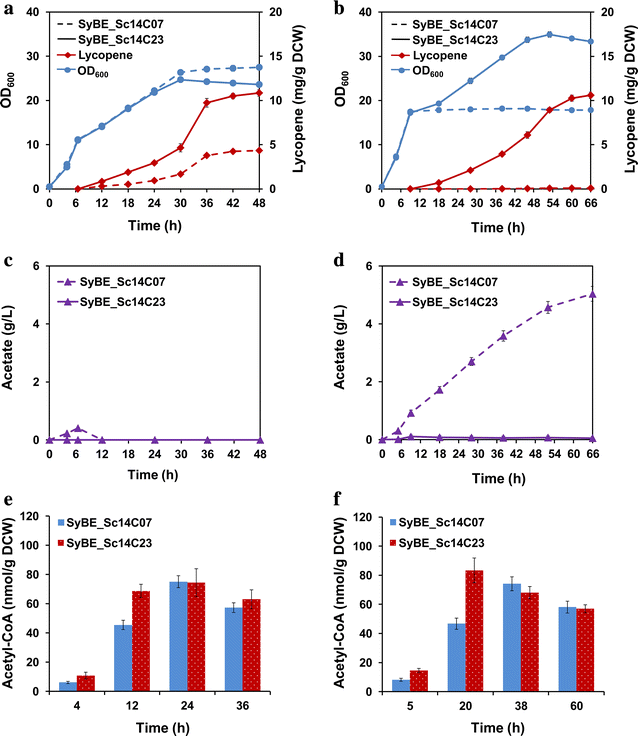
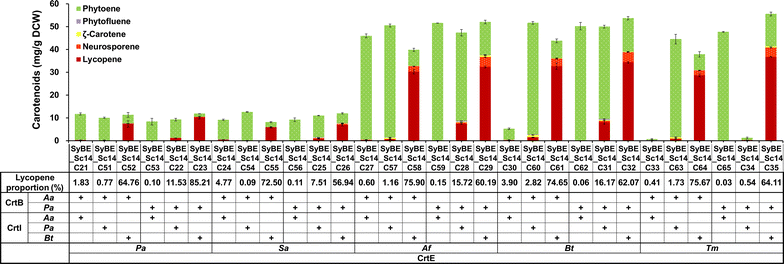
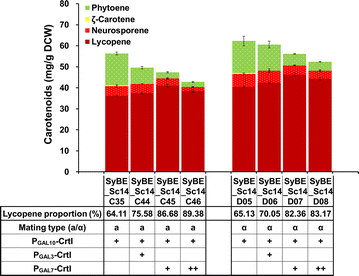
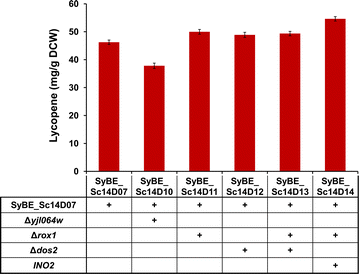
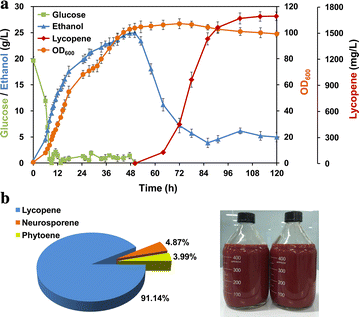
Results: In this study, lycopene biosynthesis pathway was constructed by integration of CrtE, CrtB and CrtI in S. cerevisiae CEN.PK2. When YPL062W, a distant genetic locus, was deleted, little acetate was accumulated and approximately 100 % increase in cytosolic acetyl-CoA pool was achieved relative to that in parental strain. Through screening CrtE, CrtB and CrtI from diverse species, an optimal carotenogenic enzyme combination was obtained, and CrtI from Blakeslea trispora (BtCrtI) was found to have excellent performance on lycopene production as well as lycopene proportion in carotenoid. Then, the expression level of BtCrtI was fine-tuned and the effect of cell mating types was also evaluated. Finally, potential distant genetic targets (YJL064W, ROX1, and DOS2) were deleted and a stress-responsive transcription factor INO2 was also up-regulated. Through the above modifications between host cell and carotenogenic pathway, lycopene yield was increased by approximately 22-fold (from 2.43 to 54.63 mg/g DCW). Eventually, in fed-batch fermentation, lycopene production reached 55.56 mg/g DCW, which is the highest reported yield in yeasts.
Conclusions: Saccharomyces cerevisiae was engineered to produce lycopene in this study. Through combining host engineering (distant genetic loci and cell mating types) with pathway engineering (enzyme screening and gene fine-tuning), lycopene yield was stepwise improved by 22-fold as compared to the starting strain. The highest lycopene yield (55.56 mg/g DCW) in yeasts was achieved in 5-L bioreactors. This study provides a good reference of combinatorial engineering of host cell and heterologous pathway for microbial overproduction of pharmaceutical and chemical products.
Keywords: Heterologous pathway; Lycopene; Metabolic engineering; Saccharomyces cerevisiae; Synthetic biology.
Figures
References
-
- Trikka FA, Nikolaidis A, Athanasakoglou A, Andreadelli A, Ignea C, Kotta K, Argiriou A, Kampranis SC, Makris AM. Iterative carotenogenic screens identify combinations of yeast gene deletions that enhance sclareol production. Microb Cell Fact. 2015;14:60. doi: 10.1186/s12934-015-0246-0. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

