ASC filament formation serves as a signal amplification mechanism for inflammasomes
- PMID: 27329339
- PMCID: PMC4917984
- DOI: 10.1038/ncomms11929
ASC filament formation serves as a signal amplification mechanism for inflammasomes
Erratum in
-
Corrigendum: ASC filament formation serves as a signal amplification mechanism for inflammasomes.Nat Commun. 2017 Mar 17;8:15030. doi: 10.1038/ncomms15030. Nat Commun. 2017. PMID: 28303881 Free PMC article. No abstract available.
Abstract
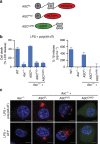
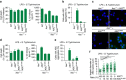
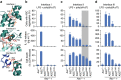
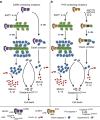
A hallmark of inflammasome activation is the ASC speck, a micrometre-sized structure formed by the inflammasome adaptor protein ASC (apoptosis-associated speck-like protein containing a CARD), which consists of a pyrin domain (PYD) and a caspase recruitment domain (CARD). Here we show that assembly of the ASC speck involves oligomerization of ASC(PYD) into filaments and cross-linking of these filaments by ASC(CARD). ASC mutants with a non-functional CARD only assemble filaments but not specks, and moreover disrupt endogenous specks in primary macrophages. Systematic site-directed mutagenesis of ASC(PYD) is used to identify oligomerization-deficient ASC mutants and demonstrate that ASC speck formation is required for efficient processing of IL-1β, but dispensable for gasdermin-D cleavage and pyroptosis induction. Our results suggest that the oligomerization of ASC creates a multitude of potential caspase-1 activation sites, thus serving as a signal amplification mechanism for inflammasome-mediated cytokine production.
Figures



References
-
- von Moltke J., Ayres J. S., Kofoed E. M., Chavarría-Smith J. & Vance R. E. Recognition of bacteria by inflammasomes. Annu. Rev. Immunol. 31, 73–106 (2013). - PubMed
-
- Philpott D. J., Sorbara M. T., Robertson S. J., Croitoru K. & Girardin S. E. NOD proteins: regulators of inflammation in health and disease. Nat. Rev. Immunol. 14, 9–23 (2014). - PubMed
-
- Martinon F., Burns K. & Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 10, 417–426 (2002). - PubMed
-
- Schroder K. & Tschopp J. The inflammasomes. Cell 140, 821–832 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

