NdhV subunit regulates the activity of type-1 NAD(P)H dehydrogenase under high light conditions in cyanobacterium Synechocystis sp. PCC 6803
- PMID: 27329499
- PMCID: PMC4916593
- DOI: 10.1038/srep28361
NdhV subunit regulates the activity of type-1 NAD(P)H dehydrogenase under high light conditions in cyanobacterium Synechocystis sp. PCC 6803
Abstract
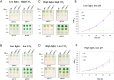
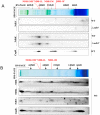
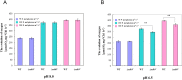
The cyanobacterial NAD(P)H dehydrogenase (NDH-1) complexes play crucial roles in variety of bioenergetic reactions. However, the regulative mechanism of NDH-1 under stressed conditions is still unclear. In this study, we detected that the NDH-1 activity is partially impaired, but the accumulation of NDH-1 complexes was little affected in the NdhV deleted mutant (ΔndhV) at low light in cyanobacterium Synechocystis sp. PCC 6803. ΔndhV grew normally at low light but slowly at high light under inorganic carbon limitation conditions (low pH or low CO2), meanwhile the activity of CO2 uptake was evidently lowered than wild type even at pH 8.0. The accumulation of NdhV in thylakoids strictly relies on the presence of the hydrophilic subcomplex of NDH-1. Furthermore, NdhV was co-located with hydrophilic subunits of NDH-1 loosely associated with the NDH-1L, NDH-1MS' and NDH-1M complexes. The level of the NdhV was significantly increased at high light and deletion of NdhV suppressed the up-regulation of NDH-1 activity, causing the lowered the photosynthetic oxygen evolution at pH 6.5 and high light. These data indicate that NdhV is an intrinsic subunit of hydrophilic subcomplex of NDH-1, required for efficient operation of cyclic electron transport around photosystem I and CO2 uptake at high lights.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
-
- Mi H. L., Endo T., Schreiber U., Ogawa T. & Asada K. Electron donation from cyclic and respiratory flows to the photosynthetic intersystem chain is mediated by pyridine-nucleotide dehydrogenase in the cyanobacterium synechocystis PCC-6803. Plant and Cell Physiology 33, 1233–1237 (1992).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

