Understanding the mechanisms of faecal microbiota transplantation
- PMID: 27329806
- PMCID: PMC5909819
- DOI: 10.1038/nrgastro.2016.98
Understanding the mechanisms of faecal microbiota transplantation
Abstract
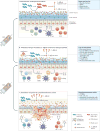
This Review summarizes mechanistic investigations in faecal microbiota transplantation (FMT), which has increasingly been adapted into clinical practice as treatment for Clostridium difficile infection (CDI) that cannot be eliminated with antibiotics alone. Administration of healthy donor faecal microbiota in this clinical situation results in its engraftment and restoration of normal gut microbial community structure and functionality. In this Review, we consider several main mechanisms for FMT effectiveness in treatment of CDI, including direct competition of C. difficile with commensal microbiota delivered by FMT, restoration of secondary bile acid metabolism in the colon and repair of the gut barrier by stimulation of the mucosal immune system. Some of these mechanistic insights suggest possibilities for developing novel, next-generation CDI therapeutics. FMT might also have potential applications for non-CDI indications. The gut can become a reservoir of other potential antibiotic-resistant pathogens under pressure of antibiotic treatments, and restoration of normal microbial community structure by FMT might be a promising approach to protect against infections with these pathogens as well. Finally, FMT could be considered for multiple chronic diseases that are associated with some form of dysbiosis. However, considerable research is needed to optimize the FMT protocols for such applications before their therapeutic promise can be evaluated.
Figures
References
-
- Laxminarayan R, et al. Antibiotic resistance — the need for global solutions. Lancet Infect Dis. 2013;13:1057–1098. - PubMed
-
- Kelly CP, LaMont JT. Clostridium difficile — more difficult than ever. N Engl J Med. 2008;359:1932–1940. - PubMed
-
- Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol. 2012;9:88–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

