Lipids, lysosomes, and autophagy
- PMID: 27330054
- PMCID: PMC5003162
- DOI: 10.1194/jlr.R067520
Lipids, lysosomes, and autophagy
Abstract
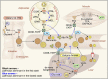
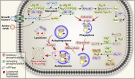
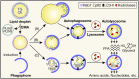
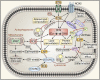
Lipids are essential components of a cell providing energy substrates for cellular processes, signaling intermediates, and building blocks for biological membranes. Lipids are constantly recycled and redistributed within a cell. Lysosomes play an important role in this recycling process that involves the recruitment of lipids to lysosomes via autophagy or endocytosis for their degradation by lysosomal hydrolases. The catabolites produced are redistributed to various cellular compartments to support basic cellular function. Several studies demonstrated a bidirectional relationship between lipids and lysosomes that regulate autophagy. While lysosomal degradation pathways regulate cellular lipid metabolism, lipids also regulate lysosome function and autophagy. In this review, we focus on this bidirectional relationship in the context of dietary lipids and provide an overview of recent evidence of how lipid-overload lipotoxicity, as observed in obesity and metabolic syndrome, impairs lysosomal function and autophagy that may eventually lead to cellular dysfunction or cell death.
Keywords: lipid metabolism; lipophagy; lipotoxicity; lysosomal dysfunction; oxidative stress; reactive oxygen species.
Copyright © 2016 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures

References
-
- Glatz J. F., Luiken J. J., and Bonen A.. 2010. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol. Rev. 90: 367–417. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

