Diesel Surrogate Fuels for Engine Testing and Chemical-Kinetic Modeling: Compositions and Properties
- PMID: 27330248
- PMCID: PMC4908839
- DOI: 10.1021/acs.energyfuels.5b02879
Diesel Surrogate Fuels for Engine Testing and Chemical-Kinetic Modeling: Compositions and Properties
Abstract
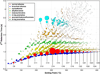
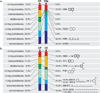
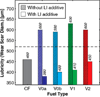
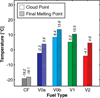
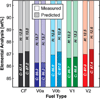
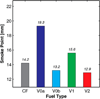
The primary objectives of this work were to formulate, blend, and characterize a set of four ultralow-sulfur diesel surrogate fuels in quantities sufficient to enable their study in single-cylinder-engine and combustion-vessel experiments. The surrogate fuels feature increasing levels of compositional accuracy (i.e., increasing exactness in matching hydrocarbon structural characteristics) relative to the single target diesel fuel upon which the surrogate fuels are based. This approach was taken to assist in determining the minimum level of surrogate-fuel compositional accuracy that is required to adequately emulate the performance characteristics of the target fuel under different combustion modes. For each of the four surrogate fuels, an approximately 30 L batch was blended, and a number of the physical and chemical properties were measured. This work documents the surrogate-fuel creation process and the results of the property measurements.
Figures










References
-
- Gieleciak R, Fairbridge C. Detailed Hydrocarbon Analysis of FACE Diesel Fuels Using Comprehensive Two-Dimensional Gas Chromatography; CDEV-2013-2065-RT. CanmetENERGY; Devon, Alberta, Canada: 2013. http://www.crcao.org/publications/advancedVehiclesFuelsLubricants/FACE/G....
-
- Gieleciak R, Oro N. A Study of FID Response Factor of GC×GC Systems for Hydrocarbon Compound Classes Existing in Diesel Fractions; CDEV-2013-1979-RT. Devon, Alberta, Canada: CanmetENERGY; 2013.
-
- Lemmon EW, Huber ML, McLinden MO. NIST Reference fluid thermodynamic and transport properties database (REFPROP), NIST Standard Reference Database 23, Version 9.1. Gaithersburg, MD, USA: National Institute of Standards and Technology; 2013.
-
- Reiter AM, Wallek T, Pfennig A, Zeymer M. Surrogate Generation and Evaluation for Diesel Fuel. Energy Fuels. 2015;29(7):4181–4192.
-
- Pitz WJ, Mueller CJ. Recent progress in the development of diesel surrogate fuels. Prog. Energy Combust. Sci. 2011;37(3):330–350.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
