Preparation, characterization, and evaluation of antitumor effect of Brucea javanica oil cationic nanoemulsions
- PMID: 27330293
- PMCID: PMC4898033
- DOI: 10.2147/IJN.S101918
Preparation, characterization, and evaluation of antitumor effect of Brucea javanica oil cationic nanoemulsions
Abstract
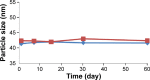
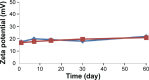
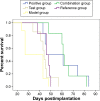
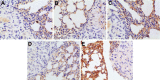
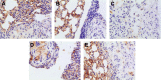
The purpose of this study was to prepare Brucea javanica oil cationic nanoemulsions (BJO-CN) with BJO as drug as well as oil phase and chitosan as cationic inducer, to explore the practical suitability of using cationic nanoemulsions for oral delivery of mixed oil, and to test its bioavailability and antitumor effect. BJO-CN was prepared by chitosan solution stirring method and then characterized physicochemically. The obtained BJO-CN had a spherical morphology with a positive zeta potential of 18.9 mV and an average particle size of 42.36 nm, showing high colloidal stability. The drug loading of BJO-CN was 91.83 mg·mL(-1), determined by high-performance liquid chromatography with precolumn derivatization. Pharmacokinetic studies revealed that, compared with BJO emulsion (BJO-E) (the dosage of BJO-CN and BJO-E was equal to 505 mg·kg(-1), calculated by oleic acid), BJO-CN exhibited a significant increase in the area under the plasma drug concentration-time curve over the period of 24 hours and relative bioavailability was 1.6-fold. Furthermore, the antitumor effect of BJO-CN in the orthotopic mouse model of lung cancer was evaluated by recording the median survival time and the weight of lung tissue with tumor, hematoxylin and eosin staining, and immunohistochemical technique. Results of anticancer experiments illustrated that, even though the administrated dosage in the BJO-CN group was half of that in the BJO-E group, BJO-CN exhibited similar antitumor effect to BJO-E. Moreover, BJO-CN had good synergistic effect in combination therapy with vinorelbine. These results suggested that cationic nanoemulsions are an effective and promising delivery system to enhance the oral bioavailability and anticancer effect of BJO.
Keywords: chitosan; combined chemotherapy; oleic acid; orthotopic mouse model of lung cancer; pharmacokinetics.
Figures










References
-
- Shi XP, Zhao HR. Research progress of Brucea javanica oil. Northwest Pharm J. 2010;25(3):240–242. Chinese.
-
- Lin HY, Wu JM, Zhang WS. Research progress of Brucea javanica oil. Chin J Exp Tradit Med Formulae. 2006;12(4):65–69. Chinese.
-
- Li JM. Stability experiment of Brucea javanica oil oral liquid. Shaanxi J Tradit Chin Med. 2006;27(2):231–232. Chinese.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

