Fluorescent Sterols and Cholesteryl Esters as Probes for Intracellular Cholesterol Transport
- PMID: 27330304
- PMCID: PMC4902042
- DOI: 10.4137/LPI.S31617
Fluorescent Sterols and Cholesteryl Esters as Probes for Intracellular Cholesterol Transport
Abstract
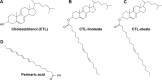
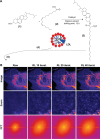
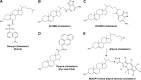
Cholesterol transport between cellular organelles comprised vesicular trafficking and nonvesicular exchange; these processes are often studied by quantitative fluorescence microscopy. A major challenge for using this approach is producing analogs of cholesterol with suitable brightness and structural and chemical properties comparable with those of cholesterol. This review surveys currently used fluorescent sterols with respect to their behavior in model membranes, their photophysical properties, as well as their transport and metabolism in cells. In the first part, several intrinsically fluorescent sterols, such as dehydroergosterol or cholestatrienol, are discussed. These polyene sterols (P-sterols) contain three conjugated double bonds in the steroid ring system, giving them slight fluorescence in ultraviolet light. We discuss the properties of P-sterols relative to cholesterol, outline their chemical synthesis, and explain how to image them in living cells and organisms. In particular, we show that P-sterol esters inserted into low-density lipoprotein can be tracked in the fibroblasts of Niemann-Pick disease using high-resolution deconvolution microscopy. We also describe fluorophore-tagged cholesterol probes, such as BODIPY-, NBD-, Dansyl-, or Pyrene-tagged cholesterol, and eventual esters of these analogs. Finally, we survey the latest developments in the synthesis and use of alkyne cholesterol analogs to be labeled with fluorophores by click chemistry and discuss the potential of all approaches for future applications.
Keywords: endocytosis; fluorescent sterols; imaging; metabolism; optical microscopy; sterol trafficking.
Figures

Similar articles
-
Live-cell imaging of new polyene sterols for improved analysis of intracellular cholesterol transport.J Microsc. 2018 Jul;271(1):36-48. doi: 10.1111/jmi.12691. Epub 2018 Mar 8. J Microsc. 2018. PMID: 29516493
-
Synthesis and Live-Cell Imaging of Fluorescent Sterols for Analysis of Intracellular Cholesterol Transport.Methods Mol Biol. 2017;1583:111-140. doi: 10.1007/978-1-4939-6875-6_10. Methods Mol Biol. 2017. PMID: 28205171
-
Fluorescent sterols as tools in membrane biophysics and cell biology.Chem Phys Lipids. 2007 Mar;146(1):1-25. doi: 10.1016/j.chemphyslip.2006.12.004. Epub 2006 Dec 30. Chem Phys Lipids. 2007. PMID: 17241621 Review.
-
Potential of BODIPY-cholesterol for analysis of cholesterol transport and diffusion in living cells.Chem Phys Lipids. 2016 Jan;194:12-28. doi: 10.1016/j.chemphyslip.2015.08.007. Epub 2015 Aug 17. Chem Phys Lipids. 2016. PMID: 26291493 Review.
-
Following intracellular cholesterol transport by linear and non-linear optical microscopy of intrinsically fluorescent sterols.Curr Pharm Biotechnol. 2012 Feb;13(2):303-18. doi: 10.2174/138920112799095301. Curr Pharm Biotechnol. 2012. PMID: 21470123 Review.
Cited by
-
Stereospecific Properties and Intracellular Transport of Novel Intrinsically Fluorescent Neurosteroids.ACS Chem Neurosci. 2024 Dec 4;15(23):4322-4336. doi: 10.1021/acschemneuro.4c00571. Epub 2024 Nov 22. ACS Chem Neurosci. 2024. PMID: 39574303
-
Dynamic Mode Decomposition of Multiphoton and Stimulated Emission Depletion Microscopy Data for Analysis of Fluorescent Probes in Cellular Membranes.Sensors (Basel). 2024 Mar 25;24(7):2096. doi: 10.3390/s24072096. Sensors (Basel). 2024. PMID: 38610307 Free PMC article.
-
Advances on the Transfer of Lipids by Lipid Transfer Proteins.Trends Biochem Sci. 2017 Jul;42(7):516-530. doi: 10.1016/j.tibs.2017.05.001. Epub 2017 Jun 1. Trends Biochem Sci. 2017. PMID: 28579073 Free PMC article. Review.
-
Interleaflet Coupling, Pinning, and Leaflet Asymmetry-Major Players in Plasma Membrane Nanodomain Formation.Front Cell Dev Biol. 2017 Jan 10;4:155. doi: 10.3389/fcell.2016.00155. eCollection 2016. Front Cell Dev Biol. 2017. PMID: 28119914 Free PMC article. Review.
-
Development and characterization of fluorescent cholesteryl probes with enhanced solvatochromic and pH-sensitive properties for live-cell imaging.Sci Rep. 2024 Dec 28;14(1):30777. doi: 10.1038/s41598-024-80958-2. Sci Rep. 2024. PMID: 39730504 Free PMC article.
References
-
- Behrman EJ, Gopalan V. Cholesterol and plants. J Chem Educ. 2005;82:1791–1793.
-
- Schroeder F, Barenholz Y, Gratton E, Thompson TE. A fluorescence study of dehydroergosterol in phosphatidylcholine bilayer vesicles. Biochemistry. 1987;26:2441–2448. - PubMed
-
- Marrink SJ, de Vries AH, Harroun TA, Katsaras J, Wassall SR. Cholesterol shows preference for the interior of polyunsaturated lipid membranes. J Am Chem Soc. 2008;130:10–11. - PubMed
-
- Aittoniemi J, Róg T, Niemelä P, Pasenkiewicz-Gierula M, Karttunen M, Vattulainen I. Tilt: major factor in sterols’ ordering capability in membranes. J Phys Chem B. 2006;110:25562–25564. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

