β-Cell regeneration through the transdifferentiation of pancreatic cells: Pancreatic progenitor cells in the pancreas
- PMID: 27330712
- PMCID: PMC4847880
- DOI: 10.1111/jdi.12475
β-Cell regeneration through the transdifferentiation of pancreatic cells: Pancreatic progenitor cells in the pancreas
Abstract
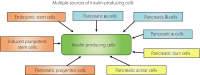
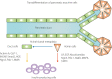
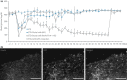
Pancreatic progenitor cell research has been in the spotlight, as these cells have the potential to replace pancreatic β-cells for the treatment of type 1 and 2 diabetic patients with the absence or reduction of pancreatic β-cells. During the past few decades, the successful treatment of diabetes through transplantation of the whole pancreas or isolated islets has nearly been achieved. However, novel sources of pancreatic islets or insulin-producing cells are required to provide sufficient amounts of donor tissues. To overcome this limitation, the use of pancreatic progenitor cells is gaining more attention. In particular, pancreatic exocrine cells, such as duct epithelial cells and acinar cells, are attractive candidates for β-cell regeneration because of their differentiation potential and pancreatic lineage characteristics. It has been assumed that β-cell neogenesis from pancreatic progenitor cells could occur in pancreatic ducts in the postnatal stage. Several studies have shown that insulin-producing cells can arise in the duct tissue of the adult pancreas. Acinar cells also might have the potential to differentiate into insulin-producing cells. The present review summarizes recent progress in research on the transdifferentiation of pancreatic exocrine cells into insulin-producing cells, especially duct and acinar cells.
Keywords: Pancreatic progenitor cells; Transdifferentiation; β‐Cell regeneration.
Figures
Similar articles
-
Pancreatic stem/progenitor cells for the treatment of diabetes.Rev Diabet Stud. 2010 Summer;7(2):105-11. doi: 10.1900/RDS.2010.7.105. Epub 2010 Aug 10. Rev Diabet Stud. 2010. PMID: 21060969 Free PMC article. Review.
-
Regenerating pancreatic beta-cells: plasticity of adult pancreatic cells and the feasibility of in-vivo neogenesis.Curr Opin Organ Transplant. 2010 Feb;15(1):79-85. doi: 10.1097/MOT.0b013e3283344932. Curr Opin Organ Transplant. 2010. PMID: 19907327 Free PMC article. Review.
-
Debates in Pancreatic Beta Cell Biology: Proliferation Versus Progenitor Differentiation and Transdifferentiation in Restoring β Cell Mass.Front Endocrinol (Lausanne). 2021 Aug 6;12:722250. doi: 10.3389/fendo.2021.722250. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34421829 Free PMC article. Review.
-
Making β(-like)-cells from exocrine pancreas.Diabetes Obes Metab. 2016 Sep;18 Suppl 1:144-51. doi: 10.1111/dom.12725. Diabetes Obes Metab. 2016. PMID: 27615144 Review.
-
Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth.Proc Natl Acad Sci U S A. 2008 Dec 16;105(50):19915-9. doi: 10.1073/pnas.0805803105. Epub 2008 Dec 3. Proc Natl Acad Sci U S A. 2008. PMID: 19052237 Free PMC article.
Cited by
-
Advancements in transplantation therapy for diabetes: Pancreas, islet and stem cell.J Diabetes Investig. 2021 Feb;12(2):143-145. doi: 10.1111/jdi.13358. Epub 2020 Aug 27. J Diabetes Investig. 2021. PMID: 32654418 Free PMC article.
-
Advanced therapy to cure diabetes: mission impossible is now possible?Front Cell Dev Biol. 2024 Nov 19;12:1484859. doi: 10.3389/fcell.2024.1484859. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39629270 Free PMC article. Review.
-
MicroRNA-9 modified bone marrow-derived mesenchymal stem cells (BMSCs) repair severe acute pancreatitis (SAP) via inducing angiogenesis in rats.Stem Cell Res Ther. 2018 Oct 25;9(1):282. doi: 10.1186/s13287-018-1022-y. Stem Cell Res Ther. 2018. Retraction in: Stem Cell Res Ther. 2023 Jul 28;14(1):186. doi: 10.1186/s13287-023-03419-z. PMID: 30359310 Free PMC article. Retracted.
-
Crosstalk Communications Between Islets Cells and Insulin Target Tissue: The Hidden Face of Iceberg.Front Endocrinol (Lausanne). 2022 Feb 3;13:836344. doi: 10.3389/fendo.2022.836344. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35185804 Free PMC article. Review.
-
Engineered tools to study endocrine dysfunction of pancreas.Biophys Rev (Melville). 2024 Oct 22;5(4):041303. doi: 10.1063/5.0220396. eCollection 2024 Dec. Biophys Rev (Melville). 2024. PMID: 39449867 Review.
References
-
- Shapiro AM, Lakey JR, Ryan EA, et al Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid‐free immunosuppressive regimen. N Engl J Med 2000; 343: 230–238. - PubMed
-
- Noguchi H, Iwanaga Y, Okitsu T, et al Evaluation of islet transplantation from non‐heart beating donors. Am J Transplant 2006; 6: 2476–2482. - PubMed
-
- Noguchi H, Ikemoto T, Naziruddin B, et al Iodixanol controlled density gradient during islet purification improves recovery rate in human islet isolation. Transplantation 2009; 87: 1629–1635. - PubMed
-
- Ricordi C, Strom TB. Clinical islet transplantation: advances and immunological challenges. Nat Rev Immuunol 2004; 4: 259–268. - PubMed
-
- Hering BJ, Kandaswamy R, Ansite JD, et al Single donor, marginal‐dose islet transplantation in patients with type 1 diabetes. JAMA 2005; 293: 830–835. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

