Progression-free and overall survival in ovarian cancer patients treated with CVac, a mucin 1 dendritic cell therapy in a randomized phase 2 trial
- PMID: 27330807
- PMCID: PMC4915201
- DOI: 10.1186/s40425-016-0137-x
Progression-free and overall survival in ovarian cancer patients treated with CVac, a mucin 1 dendritic cell therapy in a randomized phase 2 trial
Abstract
Background: CAN-003 was a randomized, open-label, Phase 2 trial evaluating the safety, efficacy and immune outcomes of CVac, a mucin 1 targeted-dendritic cell (DC) treatment as a maintenance therapy to patients with epithelial ovarian cancer (EOC).
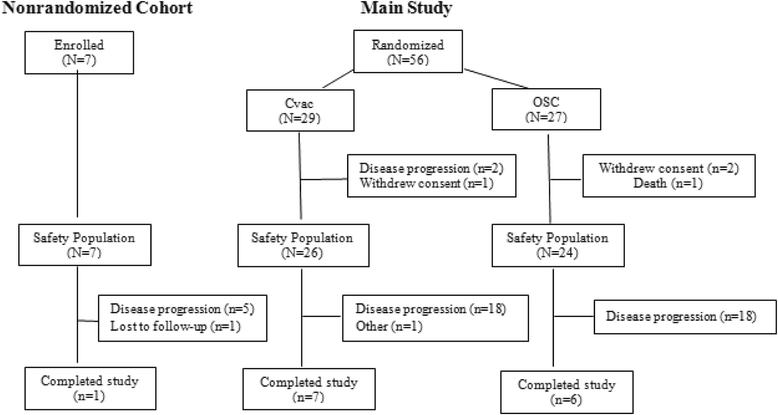
Methods: Patients (n = 56) in first (CR1) or second clinical remission (CR2) were randomized (1:1) to standard of care (SOC) observation or CVac maintenance treatment. Ten doses were administered over 56 weeks. Both groups were followed for progression-free survival (PFS) and overall survival (OS).
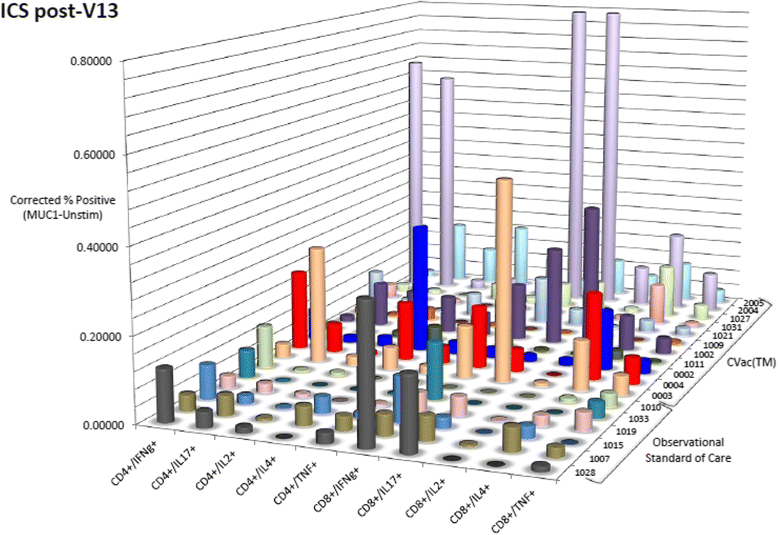
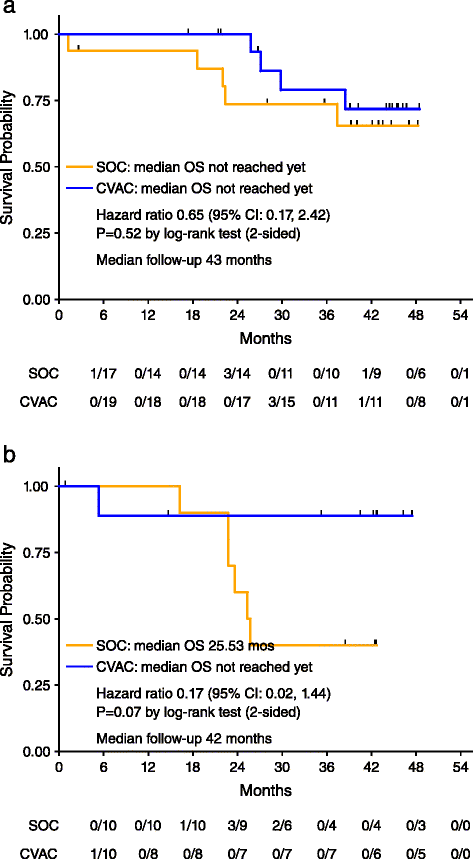
Results: Fifty-six patients were randomized: 27 to SOC and 29 to CVac. Therapy was safe with only seven patients with Grade 3-4 treatment-emergent adverse events. A variable but measurable mucin 1 T cell-specific response was induced in all CVac-treated and some standard of care (SOC) patients. Progression free survival (PFS) was not significantly longer in the treated group compared to SOC group (13 vs. 9 months, p = 0.36, hazard ratio [HR] = 0.73). Analysis by remission status showed in the CR1 subgroup a median PFS of 18 months (SOC) vs. 13 months (CVac); p = 0.69 (HR = 1.18; CI 0.52-2.71). However CR2 patients showed a longer median PFS in the CVac-treated group (median PFS not yet reached, >13 vs. 5 months; p = 0.04, HR = 0.32 CI). OS for CR2 patients at 42 months of follow-up showed a difference of 26 months for SOC vs. > 42 months for CVac-treated (as median OS had not been reached; HR = 0.17 (CI 0.02-1.4) with a p = 0.07).
Conclusions: CVac, a mucin 1-dendritic cell maintenance treatment was safe and well tolerated in ovarian cancer patients. A variable but observed CVac-derived, mucin 1-specific T cell response was measured. Notably, CR2 patients showed an improved PFS and lengthened OS. Further studies in CR2 ovarian cancer patients are warranted (NCT01068509).
Trial registration: NCT01068509. Study Initiation Date (first patient screened): 20 July 2010. Study Completion Date (last patient observation): 20 August 2013, the last patient observation for progression-free survival; 29 April 2015, the last patient was documented regarding overall survival.
Keywords: Dendritic cells; Immunotherapy; Maintenance; Mucin 1; Ovarian cancer.
Figures

References
-
- Ozols RF, Rubin SC, Thomas G, et al. Epithelial ovarian cancer. In: Hoskins WJ, Perez CA, Young RC, et al., editors. Principles and practice of gynecologic oncology. 4. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 919–922.
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
