DTI-based response-driven modeling of mTLE laterality
- PMID: 27330966
- PMCID: PMC4900487
- DOI: 10.1016/j.nicl.2015.10.015
DTI-based response-driven modeling of mTLE laterality
Abstract
Purpose: To develop lateralization models for distinguishing between unilateral and bilateral mesial temporal lobe epilepsy (mTLE) and determining laterality in cases of unilateral mTLE.
Background: mTLE is the most common form of medically refractory focal epilepsy. Many mTLE patients fail to demonstrate an unambiguous unilateral ictal onset. Intracranial EEG (icEEG) monitoring can be performed to establish whether the ictal origin is unilateral or truly bilateral with independent bitemporal ictal origin. However, because of the expense and risk of intracranial electrode placement, much research has been done to determine if the need for icEEG can be obviated with noninvasive neuroimaging methods, such as diffusion tensor imaging (DTI).
Methods: Fractional anisotropy (FA) was used to quantify microstructural changes reflected in the diffusivity properties of the corpus callosum, cingulum, and fornix, in a retrospective cohort of 31 patients confirmed to have unilateral (n = 24) or bilateral (n = 7) mTLE. All unilateral mTLE patients underwent resection with an Engel class I outcome. Eleven were reported to have hippocampal sclerosis on pathological analysis; nine had undergone prior icEEG. The bilateral mTLE patients had undergone icEEG demonstrating independent epileptiform activity in both right and left hemispheres. Twenty-three nonepileptic subjects were included as controls.
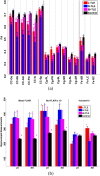
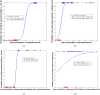
Results: In cases of right mTLE, FA showed significant differences from control in all callosal subregions, in both left and right superior cingulate subregions, and in forniceal crura. Comparison of right and left mTLE cases showed significant differences in FA of callosal genu, rostral body, and splenium and the right posteroinferior and superior cingulate subregions. In cases of left mTLE, FA showed significant differences from control only in the callosal isthmus. Significant differences in FA were identified when cases of right mTLE were compared with bilateral mTLE cases in the rostral and midbody callosal subregions and isthmus. Based on 11 FA measurements in the cingulate, callosal and forniceal subregions, a response-driven lateralization model successfully differentiated all cases (n = 54) into groups of unilateral right (n = 12), unilateral left (n = 12), and bilateral mTLE (n = 7), and nonepileptic control (23).
Conclusion: The proposed response-driven DTI biomarker is intended to lessen diagnostic ambiguity of laterality in cases of mTLE and help optimize selection of surgical candidates. Application of this model shows promise in reducing the need for invasive icEEG in prospective cases.
Keywords: Bilateral; Bitemporal; Diffusion tensor imaging; Mesial temporal lobe epilepsy; Response-driven lateralization models.
Figures

References
-
- Aghakhani Y., Liu X., Jette N., Wiebe S. Epilepsy surgery in patients with bilateral temporal lobe seizures: a systematic review. Epilepsia. 2014;55(12):1892–1901. - PubMed
-
- Akanuma N., Alarcon G., Lum F., Kissani N., Koutroumanidis M., Adachi N., Binnie C.D., Polkey C.E., Morris R.G. Lateralising value of neuropsychological protocols for presurgical assessment of temporal lobe epilepsy. Epilepsia. 2003;44:408–418. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

